当前位置:
X-MOL 学术
›
Chem. Soc. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Visible light-promoted reactions with diazo compounds: a mild and practical strategy towards free carbene intermediates.
Chemical Society Reviews ( IF 40.4 ) Pub Date : 2020-08-28 , DOI: 10.1039/d0cs00224k Zhen Yang 1 , Mateus L Stivanin 2 , Igor D Jurberg 2 , Rene M Koenigs 1
Chemical Society Reviews ( IF 40.4 ) Pub Date : 2020-08-28 , DOI: 10.1039/d0cs00224k Zhen Yang 1 , Mateus L Stivanin 2 , Igor D Jurberg 2 , Rene M Koenigs 1
Affiliation
|
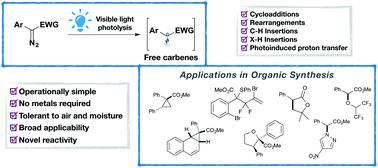
Carbenes are important intermediates in organic chemistry and have been widely applied in various types of organic reactions, ranging from cycloaddition reactions and sigmatropic rearrangements to C–H functionalizations, thus allowing the rapid construction of densely functionalized molecules. Over the past decades, remarkable progress has been achieved in metal-catalyzed carbene transfer reactions. Nevertheless, realizing these transformations under milder and/or greener conditions is still highly desirable. Only recently, visible light-promoted carbene transfer reactions of diazo compounds via free carbene intermediates have emerged as a practical, mild and powerful tool. In this tutorial review, we summarize the latest advances in the area, aiming at providing a clear overview on reaction design, mechanistic scenarios and potential future developments.
中文翻译:

与重氮化合物的可见光促进反应:生成游离卡宾中间体的温和而实用的策略。
卡宾是有机化学中重要的中间体,已广泛应用于各种类型的有机反应中,从环加成反应和σ向重排到C–H功能化,因此可以快速构建密集功能化的分子。在过去的几十年中,在金属催化的卡宾转移反应方面已经取得了显着进展。然而,仍然非常需要在温和和/或绿色条件下实现这些转化。直到最近,重氮化合物通过可见光促进的卡宾转移反应通过游离的卡宾中间体已成为一种实用,温和且功能强大的工具。在本教程的回顾中,我们总结了该领域的最新进展,旨在提供对反应设计,机械方案和潜在的未来发展的清晰概述。
更新日期:2020-10-05
中文翻译:

与重氮化合物的可见光促进反应:生成游离卡宾中间体的温和而实用的策略。
卡宾是有机化学中重要的中间体,已广泛应用于各种类型的有机反应中,从环加成反应和σ向重排到C–H功能化,因此可以快速构建密集功能化的分子。在过去的几十年中,在金属催化的卡宾转移反应方面已经取得了显着进展。然而,仍然非常需要在温和和/或绿色条件下实现这些转化。直到最近,重氮化合物通过可见光促进的卡宾转移反应通过游离的卡宾中间体已成为一种实用,温和且功能强大的工具。在本教程的回顾中,我们总结了该领域的最新进展,旨在提供对反应设计,机械方案和潜在的未来发展的清晰概述。











































 京公网安备 11010802027423号
京公网安备 11010802027423号