当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Deprotonation of Isoxazole: A Photoelectron Imaging Study.
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-08-27 , DOI: 10.1021/acs.jpca.0c06838 Adam A Wallace 1 , Yerbolat Dauletyarov 1 , Andrei Sanov 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-08-27 , DOI: 10.1021/acs.jpca.0c06838 Adam A Wallace 1 , Yerbolat Dauletyarov 1 , Andrei Sanov 1
Affiliation
|
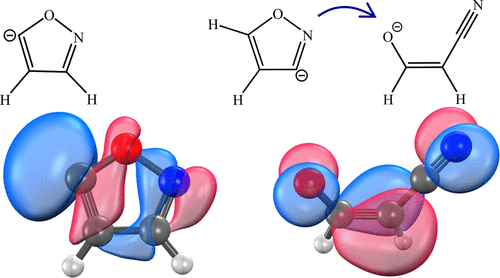
We report a photoelectron imaging study of gas-phase deprotonation of isoxazole in which spectroscopic data are compared to the results of electronic structure calculations for the anion products corresponding to each of three possible deprotonation sites. The observed photoelectron spectra are assigned to a mixture of the anion isomers. Deprotonation at the most acidic (C5) and the least acidic (C4) positions yields the respective C5- and C4-isoxazolide anions, while the reaction at the intermediate-acidity C3 site leads to a cleavage of the O–N bond and an opening of the ring in the anion. Following photodetachment, the ground states of neutral C5- and C4-isoxazolyl are assigned to be σ radicals (X2A′), while the ground-state neutral derived from the ring-open C3-anion is a π radical (X2A″). The relative intensities of the spectral bands exhibit sensitivity to the ion source conditions, giving evidence of competing and varying contributions of the dominant C5 and C3, as well as possible C4, deprotonation pathways.
中文翻译:

异恶唑的去质子化:光电子成像研究。
我们报告了异恶唑气相去质子化的光电子成像研究,其中将光谱数据与对应于三个可能的去质子化位点的阴离子产物的电子结构计算结果进行了比较。观察到的光电子能谱归属于阴离子异构体的混合物。在最强酸(C5)和最弱酸(C4)位置去质子化分别产生C5-和C4-异恶唑啉离子阴离子,而在中等酸度C3位置的反应导致O–N键的裂解和开口阴离子中的环。光解之后,中性C5-和C4-异恶唑基的基态被分配为σ自由基(X 2 A'),而源自开环C3-阴离子的基态中性则为π自由基(X 2 A'')。光谱带的相对强度显示出对离子源条件的敏感性,从而证明了主要的C5和C3以及可能的C4去质子化途径竞争和变化的贡献。
更新日期:2020-09-24
中文翻译:

异恶唑的去质子化:光电子成像研究。
我们报告了异恶唑气相去质子化的光电子成像研究,其中将光谱数据与对应于三个可能的去质子化位点的阴离子产物的电子结构计算结果进行了比较。观察到的光电子能谱归属于阴离子异构体的混合物。在最强酸(C5)和最弱酸(C4)位置去质子化分别产生C5-和C4-异恶唑啉离子阴离子,而在中等酸度C3位置的反应导致O–N键的裂解和开口阴离子中的环。光解之后,中性C5-和C4-异恶唑基的基态被分配为σ自由基(X 2 A'),而源自开环C3-阴离子的基态中性则为π自由基(X 2 A'')。光谱带的相对强度显示出对离子源条件的敏感性,从而证明了主要的C5和C3以及可能的C4去质子化途径竞争和变化的贡献。











































 京公网安备 11010802027423号
京公网安备 11010802027423号