当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development of Scalable Synthesis of 5-Butyl-4-(4-methoxyphenyl)-6-phenylpyrimidin-2-amine (WQE-134), a Dual Inhibitor of Nitric Oxide and Prostaglandin E2 Production
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2020-08-28 , DOI: 10.1021/acs.oprd.0c00324 Pavel Dosoudil 1 , Vladislav Kotek 1 , Viktor Kolman 2 , Ondřej Baszczyňski 2 , Martin Maxmilian Kaiser 2 , Zlatko Janeba 2 , Miroslav Havránek 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2020-08-28 , DOI: 10.1021/acs.oprd.0c00324 Pavel Dosoudil 1 , Vladislav Kotek 1 , Viktor Kolman 2 , Ondřej Baszczyňski 2 , Martin Maxmilian Kaiser 2 , Zlatko Janeba 2 , Miroslav Havránek 1
Affiliation
|
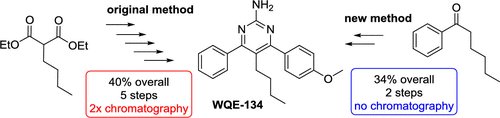
We report a scalable efficient synthesis of 5-butyl-4-(4-methoxyphenyl)-6-phenylpyrimidin-2-amine (WQE-134), a dual inhibitor of nitric oxide and prostaglandin E2 production with potent anti-inflammatory properties. The original five-step synthesis (40% overall yield) was based on two Suzuki–Miyaura reactions and required chromatographic purification of both the intermediate and final products. The novel synthetic pathway is based on cyclization of 2-butyl-1-(4-methoxyphenyl)-3-phenylpropane-1,3-dione with guanidinium methanesulfonate using Eaton’s reagent (P2O5/MsOH) at 50 °C. The two-step synthesis (34% overall yield) can be performed on a multigram to kilogram scale, and purification of the final product consists of simple extraction (dichloromethane) and crystallization (ethyl acetate and methanol).
中文翻译:

一氧化氮和前列腺素E 2生产的双重抑制剂5-丁基-4-(4-甲氧基苯基)-6-苯基嘧啶-2-胺(WQE-134)的可扩展合成方法的开发
我们报告了5-丁基-4-(4-甲氧基苯基)-6-苯基嘧啶-2-胺(WQE-134)的可扩展有效合成方法,这是一氧化氮和前列腺素E 2生产的双重抑制剂,具有强大的抗炎特性。最初的五步合成(总收率40%)基于两个Suzuki-Miyaura反应,并且需要对中间产物和最终产物进行色谱纯化。新颖的合成途径是基于使用伊顿试剂(P 2 O 5)将2-丁基-1-(4-甲氧基苯基)-3-苯基丙烷-1,3-二酮与甲磺酸胍环化/ MsOH)在50°C下。两步合成法(总收率34%)可以在几克到千克的范围内进行,最终产物的纯化包括简单的萃取(二氯甲烷)和结晶(乙酸乙酯和甲醇)。
更新日期:2020-09-20
中文翻译:

一氧化氮和前列腺素E 2生产的双重抑制剂5-丁基-4-(4-甲氧基苯基)-6-苯基嘧啶-2-胺(WQE-134)的可扩展合成方法的开发
我们报告了5-丁基-4-(4-甲氧基苯基)-6-苯基嘧啶-2-胺(WQE-134)的可扩展有效合成方法,这是一氧化氮和前列腺素E 2生产的双重抑制剂,具有强大的抗炎特性。最初的五步合成(总收率40%)基于两个Suzuki-Miyaura反应,并且需要对中间产物和最终产物进行色谱纯化。新颖的合成途径是基于使用伊顿试剂(P 2 O 5)将2-丁基-1-(4-甲氧基苯基)-3-苯基丙烷-1,3-二酮与甲磺酸胍环化/ MsOH)在50°C下。两步合成法(总收率34%)可以在几克到千克的范围内进行,最终产物的纯化包括简单的萃取(二氯甲烷)和结晶(乙酸乙酯和甲醇)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号