当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dual-Location Dual-Acid/Glutathione-Degradable Cationic Micelleplexes through Hydrophobic Modification for Enhanced Gene Silencing.
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2020-08-27 , DOI: 10.1021/acs.molpharmaceut.0c00767 Chaitra Shetty 1 , Anne Noronha 1 , Alexander Pontarelli 1 , Christopher J Wilds 1 , Jung Kwon Oh 1
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2020-08-27 , DOI: 10.1021/acs.molpharmaceut.0c00767 Chaitra Shetty 1 , Anne Noronha 1 , Alexander Pontarelli 1 , Christopher J Wilds 1 , Jung Kwon Oh 1
Affiliation
|
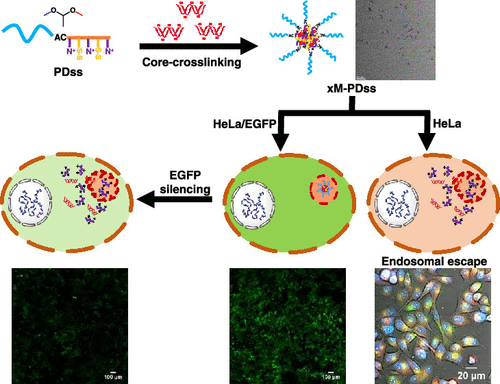
Gene therapy holds great promise for the treatment of acquired genetic disorders such as cancer with reduced side effects compared to chemotherapy. For gene therapy to be successful, it is crucial to develop efficient and nontoxic gene carriers to overcome the poor in vivo stability and low cellular uptake of nucleic acid-based therapeutic agents. Here, we report a new and versatile approach exploring a combination of hydrophobic modifications and dual-stimuli-responsive degradation (SRD) for controlled gene delivery with amphiphilic block copolymer-based nanocarriers. The block copolymer, synthesized by atom transfer radical polymerization, is designed with an acid-labile acetal linkage at the block junction and a pendant disulfide group in the hydrophobic block. The incorporation of labile linkages enables both disulfide-core-cross-linking and dual-location dual-acid/reduction-responsive degradation (DL-DSRD). Furthermore, the disulfide linkages integrated as hydrophobic moieties facilitate the nucleic acids to condense into nanometer-sized micelleplexes through electrostatic interactions of pendant dimethylamino groups with the anionic phosphate groups of the nucleic acids. Our preliminary results demonstrate that the DL-DSRD approach through hydrophobic modification is a robust platform in the development of gene delivery systems with enhanced colloidal stability, reduced cytotoxicity, and improved gene transfection efficiency.
中文翻译:

双位置双酸/谷胱甘肽可降解阳离子胶束复合物通过疏水修饰增强基因沉默。
与化学疗法相比,基因疗法在治疗获得性遗传疾病(例如癌症)方面具有巨大的希望,并且副作用更少。基因治疗要取得成功,开发高效无毒的基因载体以克服体内缺陷至关重要基于核酸的治疗剂的稳定性和低细胞摄取。在这里,我们报告了一种新的通用方法,探索疏水修饰和双刺激响应降解 (SRD) 的组合,用于使用基于两亲嵌段共聚物的纳米载体进行受控基因传递。通过原子转移自由基聚合合成的嵌段共聚物设计为在嵌段连接处具有酸不稳定的缩醛键,在疏水嵌段中具有侧链二硫键。不稳定键的结合能够实现二硫键-核心-交联和双位置双酸/还原响应性降解 (DL-DSRD)。此外,通过二甲氨基侧基与核酸的阴离子磷酸基团的静电相互作用,作为疏水部分整合的二硫键促进核酸凝聚成纳米尺寸的胶束复合体。我们的初步结果表明,通过疏水修饰的 DL-DSRD 方法是开发具有增强胶体稳定性、降低细胞毒性和提高基因转染效率的基因递送系统的强大平台。
更新日期:2020-10-05
中文翻译:

双位置双酸/谷胱甘肽可降解阳离子胶束复合物通过疏水修饰增强基因沉默。
与化学疗法相比,基因疗法在治疗获得性遗传疾病(例如癌症)方面具有巨大的希望,并且副作用更少。基因治疗要取得成功,开发高效无毒的基因载体以克服体内缺陷至关重要基于核酸的治疗剂的稳定性和低细胞摄取。在这里,我们报告了一种新的通用方法,探索疏水修饰和双刺激响应降解 (SRD) 的组合,用于使用基于两亲嵌段共聚物的纳米载体进行受控基因传递。通过原子转移自由基聚合合成的嵌段共聚物设计为在嵌段连接处具有酸不稳定的缩醛键,在疏水嵌段中具有侧链二硫键。不稳定键的结合能够实现二硫键-核心-交联和双位置双酸/还原响应性降解 (DL-DSRD)。此外,通过二甲氨基侧基与核酸的阴离子磷酸基团的静电相互作用,作为疏水部分整合的二硫键促进核酸凝聚成纳米尺寸的胶束复合体。我们的初步结果表明,通过疏水修饰的 DL-DSRD 方法是开发具有增强胶体稳定性、降低细胞毒性和提高基因转染效率的基因递送系统的强大平台。











































 京公网安备 11010802027423号
京公网安备 11010802027423号