当前位置:
X-MOL 学术
›
Environ. Sci. Technol. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Quantitative Determination of Hydroxymethanesulfonate (HMS) Using Ion Chromatography and UHPLC-LTQ-Orbitrap Mass Spectrometry: A Missing Source of Sulfur during Haze Episodes in Beijing
Environmental Science & Technology Letters ( IF 8.9 ) Pub Date : 2020-08-27 , DOI: 10.1021/acs.estlett.0c00528 Lianfang Wei 1 , Pingqing Fu 2 , Xueshun Chen 1 , Na An 3 , Siyao Yue 1, 4 , Hong Ren 2 , Wanyu Zhao 1, 4 , Qiaorong Xie 1, 4 , Yele Sun 1 , Quan-Fei Zhu 3 , Zifa Wang 1, 4, 5 , Yu-Qi Feng 3
Environmental Science & Technology Letters ( IF 8.9 ) Pub Date : 2020-08-27 , DOI: 10.1021/acs.estlett.0c00528 Lianfang Wei 1 , Pingqing Fu 2 , Xueshun Chen 1 , Na An 3 , Siyao Yue 1, 4 , Hong Ren 2 , Wanyu Zhao 1, 4 , Qiaorong Xie 1, 4 , Yele Sun 1 , Quan-Fei Zhu 3 , Zifa Wang 1, 4, 5 , Yu-Qi Feng 3
Affiliation
|
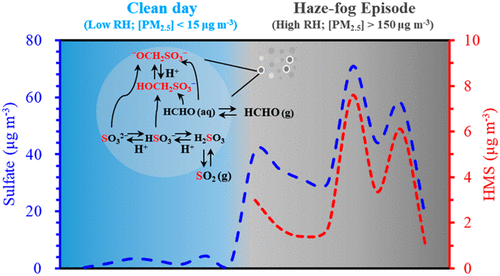
Hydroxymethanesulfonate (HMS), the product of HSO3– and SO32– reacting with dissolved formaldehyde in fog, cloud, and aerosol liquid water, has been suggested to be an important source of particulate sulfur during haze events. However, there have been limited studies of high concentrations of HMS in atmospheric aerosol. We report here two feasible analytical methods for the identification of HMS using ion chromatography and UHPLC-LTQ-Orbitrap mass spectrometry. The concentrations of HMS in fine particles in wintertime Beijing ranged from below the detection limit to 7.3 μg m–3 (with an average at 2.0 ± 2.1 μg m–3). Increased concentrations of HMS and sulfate were associated with the occurrence of fog. The results reveal that HMS may be a potential S(IV) reservoir and an effective tracer of aqueous-phase chemistry. The atmospheric aqueous-phase chemistry promoted the rapid conversion of S(IV) under haze–fog conditions. The kinetic modeling results suggest that the aqueous pH should be taken into account when exploring the mechanism of HMS formation in atmospheric sulfur chemistry.
更新日期:2020-10-13











































 京公网安备 11010802027423号
京公网安备 11010802027423号