当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Phase Behavior in Rhombohedral NaSiCON Electrolytes and Electrodes
Chemistry of Materials ( IF 7.2 ) Pub Date : 2020-08-27 , DOI: 10.1021/acs.chemmater.0c02695 Zeyu Deng 1 , Gopalakrishnan Sai Gautam 2 , Sanjeev Krishna Kolli 3 , Jean-Noël Chotard 4, 5 , Anthony K. Cheetham 3, 6 , Christian Masquelier 4, 5 , Pieremanuele Canepa 6
Chemistry of Materials ( IF 7.2 ) Pub Date : 2020-08-27 , DOI: 10.1021/acs.chemmater.0c02695 Zeyu Deng 1 , Gopalakrishnan Sai Gautam 2 , Sanjeev Krishna Kolli 3 , Jean-Noël Chotard 4, 5 , Anthony K. Cheetham 3, 6 , Christian Masquelier 4, 5 , Pieremanuele Canepa 6
Affiliation
|
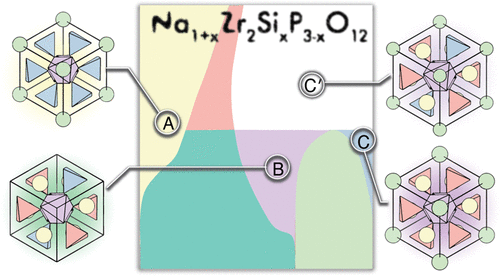
The replacement of the presently used liquid electrolytes by a nonflammable solid electrolytes is an important avenue to create safer batteries. The natrium superionic CONductor (NaSiCON) Na1+xZr2SixP3–xO12 (0 ≤ x ≤ 3) that displays high bulk ionic conductivity and good stability toward other NaSiCON-based electrodes is a good solid electrolyte in NaSiCON-based batteries. Despite the sizeable share of research on Na1+xZr2SixP3–xO12, the structural and thermodynamic properties of NaSiCON require better understanding for more efficient synthesis and optimization as a solid electrolyte, which often follows chemical intuition. Here, we analyze the thermodynamic properties of the rhombohedral NaSiCON electrolyte by constructing the Na1+xZr2SixP3–xO12 phase diagram, based on density functional theory calculations, a cluster expansion framework, and Monte Carlo simulations. Specifically, we build the phase diagram as a function of temperature and composition (0 ≤ x ≤ 3) for the high-temperature rhombohedral structure, which has been also observed in several positive electrode materials, such as Na3Ti2(PO4)3, Na3V2(PO4)3, Na3Cr2(PO4)3, and Na3Fe2(PO4)3. Through the phase diagram, we identify the concentration domains providing the highest Na+-ion conductivity and previously unreported phase-separation behavior across three different single-phase regions. Furthermore, we note the similarities in the phase behavior between Na1+xZr2SixP3–xO12 and other NaSiCON-based monotransition metal electrodes and discuss the potential competition between thermodynamics and kinetics in experimentally observed phase separation. Our work is an important addition in understanding the thermodynamics of NaSiCON-based materials and in the development of inexpensive Na-ion batteries. From our results, we propose that the addition of SiO44– moieties to single-transition metal NaSiCON-phosphate-based electrodes will significantly slow the kinetics toward phase separation.
中文翻译:

菱形NaSiCON电解质和电极中的相行为
用不可燃的固体电解质代替当前使用的液体电解质是制造更安全的电池的重要途径。的钠超离子导体(NASICON)的Na 1+ X的Zr 2的Si X P 3- X Ø 12(0≤ X ≤3),其显示高堆积离子电导率和朝向其它基于NASICON电极良好的稳定性是在NASICON良好的固体电解质型电池。尽管在Na 1+ x Zr 2 Si x P 3– x O 12方面的研究份额很大因此,需要更好地理解NaSiCON的结构和热力学性质,以便更有效地合成和优化作为固体电解质的固体电解质,而这通常遵循化学直觉。在这里,我们基于密度泛函理论计算,簇扩展框架和蒙特卡洛模拟,通过构建Na 1+ x Zr 2 Si x P 3– x O 12相图来分析菱形NaSiCON电解质的热力学性质。具体来说,我们根据温度和成分(0≤x≤3)的高温菱形体结构,这在几种正极材料中也观察到,例如Na 3 Ti 2(PO 4)3,Na 3 V 2(PO 4)3,Na 3 Cr 2(PO 4)3和Na 3 Fe 2(PO 4)3。通过相图,我们确定了提供最高Na +的浓度域离子电导率和以前未报告的跨三个不同单相区域的相分离行为。此外,我们注意到Na 1+ x Zr 2 Si x P 3– x O 12与其他基于NaSiCON的单过渡金属电极之间的相行为相似,并讨论了在实验观察到的相分离中热力学和动力学之间的潜在竞争。我们的工作对理解基于NaSiCON的材料的热力学以及开发廉价的Na离子电池是重要的补充。根据我们的结果,我们建议添加SiO 4 4 – 单过渡金属NaSiCON-磷酸盐基电极的基团将大大减慢向相分离的动力学。
更新日期:2020-09-22
中文翻译:

菱形NaSiCON电解质和电极中的相行为
用不可燃的固体电解质代替当前使用的液体电解质是制造更安全的电池的重要途径。的钠超离子导体(NASICON)的Na 1+ X的Zr 2的Si X P 3- X Ø 12(0≤ X ≤3),其显示高堆积离子电导率和朝向其它基于NASICON电极良好的稳定性是在NASICON良好的固体电解质型电池。尽管在Na 1+ x Zr 2 Si x P 3– x O 12方面的研究份额很大因此,需要更好地理解NaSiCON的结构和热力学性质,以便更有效地合成和优化作为固体电解质的固体电解质,而这通常遵循化学直觉。在这里,我们基于密度泛函理论计算,簇扩展框架和蒙特卡洛模拟,通过构建Na 1+ x Zr 2 Si x P 3– x O 12相图来分析菱形NaSiCON电解质的热力学性质。具体来说,我们根据温度和成分(0≤x≤3)的高温菱形体结构,这在几种正极材料中也观察到,例如Na 3 Ti 2(PO 4)3,Na 3 V 2(PO 4)3,Na 3 Cr 2(PO 4)3和Na 3 Fe 2(PO 4)3。通过相图,我们确定了提供最高Na +的浓度域离子电导率和以前未报告的跨三个不同单相区域的相分离行为。此外,我们注意到Na 1+ x Zr 2 Si x P 3– x O 12与其他基于NaSiCON的单过渡金属电极之间的相行为相似,并讨论了在实验观察到的相分离中热力学和动力学之间的潜在竞争。我们的工作对理解基于NaSiCON的材料的热力学以及开发廉价的Na离子电池是重要的补充。根据我们的结果,我们建议添加SiO 4 4 – 单过渡金属NaSiCON-磷酸盐基电极的基团将大大减慢向相分离的动力学。











































 京公网安备 11010802027423号
京公网安备 11010802027423号