当前位置:
X-MOL 学术
›
Z. Anorg. Allg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Metal‐ion‐dependent, Solvent‐mediated Structural Transformation and Simultaneous Partial Transmetalation of an srs Framework into Desulfurization‐efficient Co‐Cu‐HKUST‐1
Zeitschrift für anorganische und allgemeine Chemie ( IF 1.1 ) Pub Date : 2020-08-28 , DOI: 10.1002/zaac.202000007 Shu‐Qin Liu 1 , Jian‐Jun Zhang 1 , En‐Pei Tan 1 , Huajun Andrew Zhou 2 , Fu‐Ping Tian 1 , Ye Yao 1
Zeitschrift für anorganische und allgemeine Chemie ( IF 1.1 ) Pub Date : 2020-08-28 , DOI: 10.1002/zaac.202000007 Shu‐Qin Liu 1 , Jian‐Jun Zhang 1 , En‐Pei Tan 1 , Huajun Andrew Zhou 2 , Fu‐Ping Tian 1 , Ye Yao 1
Affiliation
|
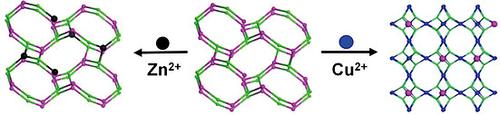
[Co2(BTC)(Cl)(DMA)3] (1) (BTC3– = benzene‐1,3,5‐tricarboxylate, DMA = N,N‐dimethylacetamide) obtained from the reaction between Co2+ and H3BTC in DMA features a three‐dimensional srs framework built of 3‐connected {Co2(COO)3} as secondary building units and BTC3– as spacers. When exposed to DMA solution of Cu(NO3)2, 1 was progressively transformed into the first heterometallic Co‐Cu‐HKUST‐1 ([Co0.14Cu2.86(BTC)2]) (2) of such kind via unusually solvent‐mediated structural transformation and simultaneous partial transmetalation. While the mechanism for such conversion is proposed based on systematic studies, 2 was revealed to be an equally efficient desulfurization adsorbent as the homometallic Cu‐HKUST‐1 in removing thiophene (0.142 mmol S per gram of adsorbent). However, when exposed to Zn(NO3)2 solution in DMA for longer time, 1 retained its framework with limited metal‐ion exchange, resulting in the formation of [Co1.93Zn0.07(BTC)(Cl)(DMA)3] (3). Possible reasons responsible for the formation of 2 and 3 through different routes could be due to the less solubility and more thermodynamic stability of 2 in comparison with those of 1, and the different coordination geometries which Co2+, Zn2+ and Cu2+ prefer.
中文翻译:

依赖金属离子,溶剂介导的结构转变和srs框架的同时部分重金属转化为脱硫效率高的Co-Cu-HKUST-1
由Co 2+与H之间的反应获得的[Co 2(BTC)(Cl)(DMA)3 ](1)(BTC 3– =苯-1,3,5-三羧酸酯,DMA = N,N-二甲基乙酰胺)3 DMA中的BTC具有一个三维srs框架,该框架由3个连通的{Co 2(COO)3 }作为辅助建筑单元,而BTC 3–作为间隔器构建。当暴露于Cu(NO 3)2的DMA溶液中时,1逐渐转化为第一个杂金属Co-Cu-HKUST-1([Co 0.14 Cu 2.86(BTC)2])(2)通过非常规的溶剂介导的结构转变和同时的部分重金属化来实现。尽管基于系统研究提出了这种转化机理,但发现2与脱金属噻吩同等金属Cu-HKUST-1是同样有效的脱硫吸附剂(0.142 mmol S /克吸附剂)。但是,当长时间暴露于DMA中的Zn(NO 3)2溶液中时,1保留了其框架,金属离子交换受到限制,导致形成了[Co 1.93 Zn 0.07(BTC)(Cl)(DMA)3 ]。 (3)。可能原因形成2和3通过不同的途径可能是由于2与1相比溶解度较低和热力学稳定性更高,并且Co 2 +,Zn 2+和Cu 2+的配位几何形状不同。
更新日期:2020-09-14
中文翻译:

依赖金属离子,溶剂介导的结构转变和srs框架的同时部分重金属转化为脱硫效率高的Co-Cu-HKUST-1
由Co 2+与H之间的反应获得的[Co 2(BTC)(Cl)(DMA)3 ](1)(BTC 3– =苯-1,3,5-三羧酸酯,DMA = N,N-二甲基乙酰胺)3 DMA中的BTC具有一个三维srs框架,该框架由3个连通的{Co 2(COO)3 }作为辅助建筑单元,而BTC 3–作为间隔器构建。当暴露于Cu(NO 3)2的DMA溶液中时,1逐渐转化为第一个杂金属Co-Cu-HKUST-1([Co 0.14 Cu 2.86(BTC)2])(2)通过非常规的溶剂介导的结构转变和同时的部分重金属化来实现。尽管基于系统研究提出了这种转化机理,但发现2与脱金属噻吩同等金属Cu-HKUST-1是同样有效的脱硫吸附剂(0.142 mmol S /克吸附剂)。但是,当长时间暴露于DMA中的Zn(NO 3)2溶液中时,1保留了其框架,金属离子交换受到限制,导致形成了[Co 1.93 Zn 0.07(BTC)(Cl)(DMA)3 ]。 (3)。可能原因形成2和3通过不同的途径可能是由于2与1相比溶解度较低和热力学稳定性更高,并且Co 2 +,Zn 2+和Cu 2+的配位几何形状不同。







































 京公网安备 11010802027423号
京公网安备 11010802027423号