当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Differences in the Performance of Allyl Based Palladium Precatalysts for Suzuki‐Miyaura Reactions
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-08-27 , DOI: 10.1002/adsc.202000987 Matthew R Espinosa 1 , Angelino Doppiu 2 , Nilay Hazari 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-08-27 , DOI: 10.1002/adsc.202000987 Matthew R Espinosa 1 , Angelino Doppiu 2 , Nilay Hazari 1
Affiliation
|
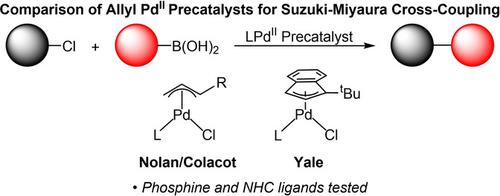
Palladium(II) precatalysts are used extensively to facilitate cross‐coupling reactions because they are bench stable and give high activity. As a result, precatalysts such as Buchwald's palladacycles, Organ's PEPPSI species, Nolan's allyl‐based complexes, and Yale's 1‐tert‐butylindenyl containing complexes, are all commercially available. Comparing the performance of the different classes of precatalysts is challenging because they are typically used under different conditions, in part because they are reduced to the active species via different pathways. However, within a particular class of precatalyst, it is easier to compare performance because they activate via similar pathways and are used under the same conditions. Here, we evaluate the activity of different allyl‐based precatalysts, such as (η3‐allyl)PdCl(L), (η3‐crotyl)PdCl(L), (η3‐cinnamyl)PdCl(L), and (η3‐1‐tert‐butylindenyl)PdCl(L) in Suzuki‐Miyaura reactions. Specifically, we evaluate precatalyst performance as the ancillary ligand (NHC or phosphine), reaction conditions, and substrates are varied. In some cases, we connect relative activity to both the mechanism of activation and the prevalence of the formation of inactive palladium(I) dimers. Additionally, we compare the performance of in situ generated precatalysts with commonly used palladium sources such as tris(dibenzylideneacetone)dipalladium(0) (Pd2dba3), bis(acetonitrile)dichloropalladium(II) (Pd(CH3CN)2Cl2), and palladium acetate. Our results provide information about which precatalyst to use under different conditions.
中文翻译:

用于 Suzuki-Miyaura 反应的烯丙基钯预催化剂的性能差异
钯 (II) 预催化剂被广泛用于促进交叉偶联反应,因为它们在工作台上稳定且具有高活性。因此,诸如 Buchwald 的钯环、Organ 的 PEPPSI 物种、Nolan 的基于烯丙基的配合物和耶鲁的 1-叔丁基茚基的配合物等预催化剂都可以在市场上买到。比较不同类别的预催化剂的性能具有挑战性,因为它们通常在不同的条件下使用,部分原因是它们通过不同的途径还原为活性物质。然而,在特定类别的预催化剂中,比较性能更容易,因为它们通过相似的途径激活并在相同的条件下使用。在这里,我们评估了不同烯丙基预催化剂的活性,3 -烯丙基)的PdCl(L),(η 3 -crotyl)的PdCl(L),(η 3 -cinnamyl)的PdCl(L),和(η 3 -1-叔-butylindenyl)的PdCl(L)在铃木-宫浦反应。具体来说,我们评估了预催化剂的性能,因为辅助配体(NHC 或膦)、反应条件和底物各不相同。在某些情况下,我们将相对活性与激活机制和非活性钯 (I) 二聚体形成的普遍性联系起来。此外,我们将原位生成的预催化剂与常用的钯源,如三(二亚苄基丙酮)二钯(0)(Pd 2 dba 3)、双(乙腈)二氯钯(II)(Pd(CH 3CN) 2 Cl 2 )和乙酸钯。我们的结果提供了有关在不同条件下使用哪种预催化剂的信息。
更新日期:2020-08-27
中文翻译:

用于 Suzuki-Miyaura 反应的烯丙基钯预催化剂的性能差异
钯 (II) 预催化剂被广泛用于促进交叉偶联反应,因为它们在工作台上稳定且具有高活性。因此,诸如 Buchwald 的钯环、Organ 的 PEPPSI 物种、Nolan 的基于烯丙基的配合物和耶鲁的 1-叔丁基茚基的配合物等预催化剂都可以在市场上买到。比较不同类别的预催化剂的性能具有挑战性,因为它们通常在不同的条件下使用,部分原因是它们通过不同的途径还原为活性物质。然而,在特定类别的预催化剂中,比较性能更容易,因为它们通过相似的途径激活并在相同的条件下使用。在这里,我们评估了不同烯丙基预催化剂的活性,3 -烯丙基)的PdCl(L),(η 3 -crotyl)的PdCl(L),(η 3 -cinnamyl)的PdCl(L),和(η 3 -1-叔-butylindenyl)的PdCl(L)在铃木-宫浦反应。具体来说,我们评估了预催化剂的性能,因为辅助配体(NHC 或膦)、反应条件和底物各不相同。在某些情况下,我们将相对活性与激活机制和非活性钯 (I) 二聚体形成的普遍性联系起来。此外,我们将原位生成的预催化剂与常用的钯源,如三(二亚苄基丙酮)二钯(0)(Pd 2 dba 3)、双(乙腈)二氯钯(II)(Pd(CH 3CN) 2 Cl 2 )和乙酸钯。我们的结果提供了有关在不同条件下使用哪种预催化剂的信息。











































 京公网安备 11010802027423号
京公网安备 11010802027423号