当前位置:
X-MOL 学术
›
Immunology
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
TLR5 activation in hepatocytes alleviates the functional suppression of intrahepatic CD8+ T cells.
Immunology ( IF 6.4 ) Pub Date : 2020-08-27 , DOI: 10.1111/imm.13251 Hu Yan 1, 2 , Maohua Zhong 1 , Jingyi Yang 1 , Jiabao Guo 1, 2 , Jie Yu 1, 2 , Yi Yang 1, 2 , Zhiyong Ma 3 , Bali Zhao 1, 2 , Yue Zhang 1, 2 , Junzhong Wang 4 , Chunchen Wu 5 , Ulf Dittmer 6 , Dongliang Yang 4 , Mengji Lu 6 , Ejuan Zhang 1 , Huimin Yan 1, 2
Immunology ( IF 6.4 ) Pub Date : 2020-08-27 , DOI: 10.1111/imm.13251 Hu Yan 1, 2 , Maohua Zhong 1 , Jingyi Yang 1 , Jiabao Guo 1, 2 , Jie Yu 1, 2 , Yi Yang 1, 2 , Zhiyong Ma 3 , Bali Zhao 1, 2 , Yue Zhang 1, 2 , Junzhong Wang 4 , Chunchen Wu 5 , Ulf Dittmer 6 , Dongliang Yang 4 , Mengji Lu 6 , Ejuan Zhang 1 , Huimin Yan 1, 2
Affiliation
|
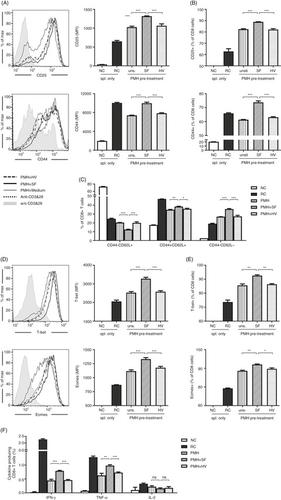
The liver is an immune‐privileged organ with a tolerogenic environment for maintaining liver homeostasis. This hepatic tolerance limits the intrahepatic CD8+ T‐cell response for eliminating infections. The tolerant microenvironment in the liver is orchestrated by liver‐specific immunoregulatory cells that can be functionally regulated by pathogen‐associated molecular patterns (PAMPs). Here, we report that flagellin, a key PAMP of gut bacteria, modulates the intrahepatic CD8+ T‐cell response by activating the TLR5 signalling pathway of hepatocytes. We found that mice treated with Salmonella‐derived recombinant flagellin (SF) by hydrodynamic injection had a significantly elevated IFN‐γ production by the intrahepatic lymphocytes in 7 days after injection. This was correlated with a reduced immune suppressive effect of primary mouse hepatocytes (PMHs) in comparison with that of PMHs from mock‐injected control mice. In vitro co‐culture of SF‐treated PMHs with splenocytes revealed that hepatocyte‐induced immune suppression is alleviated through activation of the TLR5 but not the NLRC4 signalling pathway, leading to improved activation and function of CD8+ T cells during anti‐CD3 stimulation or antigen‐specific activation. In an acute HBV replication mouse model established by co‐administration of SF together with an HBV‐replicating plasmid by hydrodynamic injection, SF significantly enhanced the intrahepatic HBV‐specific CD8+ T‐cell response against HBV surface antigen. Our results clearly showed that flagellin plays a role in modulating the intrahepatic CD8+ T‐cell response by activating the TLR5 pathway in PMHs, which suggests a potential role for gut bacteria in regulating liver immunity.
中文翻译:

肝细胞中的 TLR5 激活减轻了肝内 CD8+ T 细胞的功能抑制。
肝脏是具有免疫特权的器官,具有维持肝脏稳态的耐受环境。这种肝脏耐受性限制了消除感染的肝内 CD8 + T 细胞反应。肝脏中的耐受微环境由肝脏特异性免疫调节细胞精心策划,这些细胞可以通过病原体相关分子模式 (PAMP) 进行功能调节。在这里,我们报告鞭毛蛋白,肠道细菌的关键 PAMP,调节肝内 CD8 +T 细胞反应通过激活肝细胞的 TLR5 信号通路。我们发现,通过流体动力学注射用沙门氏菌衍生的重组鞭毛蛋白 (SF) 治疗的小鼠在注射后 7 天内肝内淋巴细胞产生的 IFN-γ 显着增加。与来自模拟注射的对照小鼠的 PMH 相比,这与原代小鼠肝细胞 (PMH) 的免疫抑制作用降低有关。SF 处理的 PMH 与脾细胞的体外共培养表明,肝细胞诱导的免疫抑制通过激活 TLR5 而不是 NLRC4 信号通路得到缓解,导致 CD8 + 的激活和功能得到改善抗 CD3 刺激或抗原特异性激活过程中的 T 细胞。在通过流体动力学注射 SF 和 HBV 复制质粒共同给药建立的急性 HBV 复制小鼠模型中,SF 显着增强了肝内 HBV 特异性 CD8 + T 细胞对 HBV 表面抗原的反应。我们的结果清楚地表明,鞭毛蛋白通过激活 PMH 中的 TLR5 途径在调节肝内 CD8 + T 细胞反应中发挥作用,这表明肠道细菌在调节肝脏免疫方面具有潜在作用。
更新日期:2020-08-27
中文翻译:

肝细胞中的 TLR5 激活减轻了肝内 CD8+ T 细胞的功能抑制。
肝脏是具有免疫特权的器官,具有维持肝脏稳态的耐受环境。这种肝脏耐受性限制了消除感染的肝内 CD8 + T 细胞反应。肝脏中的耐受微环境由肝脏特异性免疫调节细胞精心策划,这些细胞可以通过病原体相关分子模式 (PAMP) 进行功能调节。在这里,我们报告鞭毛蛋白,肠道细菌的关键 PAMP,调节肝内 CD8 +T 细胞反应通过激活肝细胞的 TLR5 信号通路。我们发现,通过流体动力学注射用沙门氏菌衍生的重组鞭毛蛋白 (SF) 治疗的小鼠在注射后 7 天内肝内淋巴细胞产生的 IFN-γ 显着增加。与来自模拟注射的对照小鼠的 PMH 相比,这与原代小鼠肝细胞 (PMH) 的免疫抑制作用降低有关。SF 处理的 PMH 与脾细胞的体外共培养表明,肝细胞诱导的免疫抑制通过激活 TLR5 而不是 NLRC4 信号通路得到缓解,导致 CD8 + 的激活和功能得到改善抗 CD3 刺激或抗原特异性激活过程中的 T 细胞。在通过流体动力学注射 SF 和 HBV 复制质粒共同给药建立的急性 HBV 复制小鼠模型中,SF 显着增强了肝内 HBV 特异性 CD8 + T 细胞对 HBV 表面抗原的反应。我们的结果清楚地表明,鞭毛蛋白通过激活 PMH 中的 TLR5 途径在调节肝内 CD8 + T 细胞反应中发挥作用,这表明肠道细菌在调节肝脏免疫方面具有潜在作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号