当前位置:
X-MOL 学术
›
J. Chem. Thermodyn.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solubility profiles of linagliptin in pure and mixed solvents: Interactions and thermodynamic parameters
The Journal of Chemical Thermodynamics ( IF 2.2 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.jct.2020.106273 Zhou Guoquan , Shao Danfeng , Shen Jian , Yang Zehui
The Journal of Chemical Thermodynamics ( IF 2.2 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.jct.2020.106273 Zhou Guoquan , Shao Danfeng , Shen Jian , Yang Zehui
|
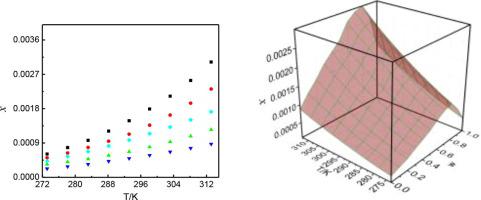
Abstract During the linagliptin process development, several related probable raw materials related and process related impurities in the range of 0.05% to 0.15% were observed. The objective of this work is to study the solubility profiles of linagliptin in pure and mixed solvents and research the thermodynamic parameters. The function of temperature and solvent composition was evaluated by some thermodynamic models. Maximum mole solubility value at 313.15 K was obtained in methanol (3.02×10-3) in pure solvents, followed by ethanol (2.31×10-3), acetonitrile (1.72×10-3), n-propanol (1.25×10-3) and isopropanol (8.74×10-4). However, in mixtures of acetonitrile (w) + ethanol (1-w), the solubility profiles increased monotonically with the increasing temperature, and increased with the increasing mass fraction (w) of acetonitrile to a maximum data at w = 0.80 and then decreased at each temperature. In order to study the role played by different modes of interaction on solubility, a multiple linear regression analysis (MLRA) involving various solvent parameters was used. The results obtained indicate that polarity/polarizability of solvent has a greater influence on the solubility of linagliptin. The calculation of thermodynamic parameters in the dissolution process indicates that the dissolution was endothermic and entropy-driven. More importantly, the change of polarity of mixed solvent is helpful for the separation of several related probable raw materials related and process related impurities during the process development.
中文翻译:

利格列汀在纯溶剂和混合溶剂中的溶解度曲线:相互作用和热力学参数
摘要 在利格列汀工艺开发过程中,观察到了几种可能的原料相关和工艺相关杂质,范围在 0.05% 至 0.15% 之间。这项工作的目的是研究利格列汀在纯溶剂和混合溶剂中的溶解度曲线并研究热力学参数。通过一些热力学模型评估了温度和溶剂组成的函数。在 313.15 K 的最大摩尔溶解度值是在纯溶剂中的甲醇 (3.02×10-3)、乙醇 (2.31×10-3)、乙腈 (1.72×10-3)、正丙醇 (1.25×10-3) 中获得的3)和异丙醇(8.74×10-4)。然而,在乙腈 (w) + 乙醇 (1-w) 的混合物中,溶解度曲线随着温度的升高而单调增加,并且随着乙腈质量分数 (w) 的增加而增加,在 w = 0.80 时达到最大值,然后在每个温度下降低。为了研究不同相互作用模式对溶解度的影响,使用了涉及各种溶剂参数的多元线性回归分析 (MLRA)。所得结果表明溶剂的极性/极化性对利格列汀的溶解度有较大影响。溶出过程中热力学参数的计算表明溶出是吸热和熵驱动的。更重要的是,混合溶剂极性的变化有助于在工艺开发过程中分离几种相关的可能原材料相关和工艺相关的杂质。为了研究不同相互作用模式对溶解度的影响,使用了涉及各种溶剂参数的多元线性回归分析 (MLRA)。所得结果表明溶剂的极性/极化性对利格列汀的溶解度有较大影响。溶出过程中热力学参数的计算表明溶出是吸热和熵驱动的。更重要的是,混合溶剂极性的变化有助于在工艺开发过程中分离几种相关的可能原材料相关和工艺相关的杂质。为了研究不同相互作用模式对溶解度的影响,使用了涉及各种溶剂参数的多元线性回归分析 (MLRA)。所得结果表明溶剂的极性/极化性对利格列汀的溶解度有较大影响。溶出过程中热力学参数的计算表明溶出是吸热和熵驱动的。更重要的是,混合溶剂极性的变化有助于在工艺开发过程中分离几种相关的可能原材料相关和工艺相关的杂质。所得结果表明溶剂的极性/极化性对利格列汀的溶解度有较大影响。溶出过程中热力学参数的计算表明溶出是吸热和熵驱动的。更重要的是,混合溶剂极性的变化有助于在工艺开发过程中分离几种相关的可能原材料相关和工艺相关的杂质。所得结果表明溶剂的极性/极化性对利格列汀的溶解度有较大影响。溶出过程中热力学参数的计算表明溶出是吸热和熵驱动的。更重要的是,混合溶剂极性的变化有助于在工艺开发过程中分离几种相关的可能原材料相关和工艺相关的杂质。
更新日期:2021-01-01
中文翻译:

利格列汀在纯溶剂和混合溶剂中的溶解度曲线:相互作用和热力学参数
摘要 在利格列汀工艺开发过程中,观察到了几种可能的原料相关和工艺相关杂质,范围在 0.05% 至 0.15% 之间。这项工作的目的是研究利格列汀在纯溶剂和混合溶剂中的溶解度曲线并研究热力学参数。通过一些热力学模型评估了温度和溶剂组成的函数。在 313.15 K 的最大摩尔溶解度值是在纯溶剂中的甲醇 (3.02×10-3)、乙醇 (2.31×10-3)、乙腈 (1.72×10-3)、正丙醇 (1.25×10-3) 中获得的3)和异丙醇(8.74×10-4)。然而,在乙腈 (w) + 乙醇 (1-w) 的混合物中,溶解度曲线随着温度的升高而单调增加,并且随着乙腈质量分数 (w) 的增加而增加,在 w = 0.80 时达到最大值,然后在每个温度下降低。为了研究不同相互作用模式对溶解度的影响,使用了涉及各种溶剂参数的多元线性回归分析 (MLRA)。所得结果表明溶剂的极性/极化性对利格列汀的溶解度有较大影响。溶出过程中热力学参数的计算表明溶出是吸热和熵驱动的。更重要的是,混合溶剂极性的变化有助于在工艺开发过程中分离几种相关的可能原材料相关和工艺相关的杂质。为了研究不同相互作用模式对溶解度的影响,使用了涉及各种溶剂参数的多元线性回归分析 (MLRA)。所得结果表明溶剂的极性/极化性对利格列汀的溶解度有较大影响。溶出过程中热力学参数的计算表明溶出是吸热和熵驱动的。更重要的是,混合溶剂极性的变化有助于在工艺开发过程中分离几种相关的可能原材料相关和工艺相关的杂质。为了研究不同相互作用模式对溶解度的影响,使用了涉及各种溶剂参数的多元线性回归分析 (MLRA)。所得结果表明溶剂的极性/极化性对利格列汀的溶解度有较大影响。溶出过程中热力学参数的计算表明溶出是吸热和熵驱动的。更重要的是,混合溶剂极性的变化有助于在工艺开发过程中分离几种相关的可能原材料相关和工艺相关的杂质。所得结果表明溶剂的极性/极化性对利格列汀的溶解度有较大影响。溶出过程中热力学参数的计算表明溶出是吸热和熵驱动的。更重要的是,混合溶剂极性的变化有助于在工艺开发过程中分离几种相关的可能原材料相关和工艺相关的杂质。所得结果表明溶剂的极性/极化性对利格列汀的溶解度有较大影响。溶出过程中热力学参数的计算表明溶出是吸热和熵驱动的。更重要的是,混合溶剂极性的变化有助于在工艺开发过程中分离几种相关的可能原材料相关和工艺相关的杂质。











































 京公网安备 11010802027423号
京公网安备 11010802027423号