当前位置:
X-MOL 学术
›
J. Chem. Thermodyn.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thermodynamic properties of stable states cerium compounds in fused 3LiCl-2KCl eutectic
The Journal of Chemical Thermodynamics ( IF 2.2 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.jct.2020.106260 Minghui Xu , Valeri Smolenski , Qi Liu , Alena Novoselova , Kewei Jiang , Jing Yu , Jingyuan Liu , Rongrong Chen , Hongsen Zhang , Milin Zhang , Jun Wang
The Journal of Chemical Thermodynamics ( IF 2.2 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.jct.2020.106260 Minghui Xu , Valeri Smolenski , Qi Liu , Alena Novoselova , Kewei Jiang , Jing Yu , Jingyuan Liu , Rongrong Chen , Hongsen Zhang , Milin Zhang , Jun Wang
|
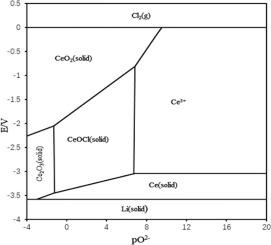
Abstract The interaction of oxygen and cerium-free ions in molten 3LiCl-2KCl eutectic in the temperature range of (723–823) K was studied by the method of potentiometric titration using YZME electrodes. Stable states of cerium compounds in the Ce–O system and the mechanism of interaction of Ce3+ with O2− ions have been established. The solubility constants of cerium oxychloride and oxide compounds at different temperatures were determined. Principal thermodynamic data of CeOCl and Ce2O3 were calculated. The Pourbaix (potential-pO2−) diagram was drawn and summarizes the properties of stable cerium species in the melt.
中文翻译:

熔融3LiCl-2KCl共晶中稳态铈化合物的热力学性质
摘要 使用YZME电极通过电位滴定法研究了(723-823) K温度范围内熔融3LiCl-2KCl共晶中氧和无铈离子的相互作用。Ce-O 体系中铈化合物的稳定状态以及 Ce3+ 与 O2− 离子相互作用的机制已经建立。测定了氯氧化铈和氧化物在不同温度下的溶解度常数。计算了 CeOCl 和 Ce2O3 的主要热力学数据。绘制了Pourbaix(电位-pO2-)图并总结了熔体中稳定铈物质的特性。
更新日期:2021-01-01
中文翻译:

熔融3LiCl-2KCl共晶中稳态铈化合物的热力学性质
摘要 使用YZME电极通过电位滴定法研究了(723-823) K温度范围内熔融3LiCl-2KCl共晶中氧和无铈离子的相互作用。Ce-O 体系中铈化合物的稳定状态以及 Ce3+ 与 O2− 离子相互作用的机制已经建立。测定了氯氧化铈和氧化物在不同温度下的溶解度常数。计算了 CeOCl 和 Ce2O3 的主要热力学数据。绘制了Pourbaix(电位-pO2-)图并总结了熔体中稳定铈物质的特性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号