Journal of Catalysis ( IF 6.5 ) Pub Date : 2020-08-28 , DOI: 10.1016/j.jcat.2020.08.023 Sadia Afrin , Praveen Bollini
|
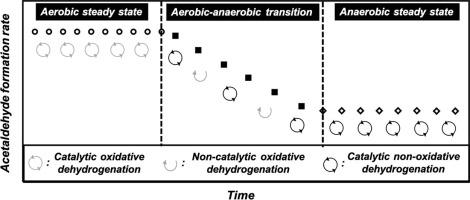
Quantitative analyses of four sets of aerobic/anaerobic ethanol conversion transients point to the evolution of a native high-surface-area cerium oxide surface that effects the reduction half of the ethanol oxidation turnover to catalyzing, exclusively, nonoxidative ethanol dehydrogenation upon complete surface reduction. Aerobic–anaerobic switches at 498 K lead to new steady state dehydrogenation rates, rather than termination of catalytic acetaldehyde formation. Concurrent termination of oxygen imbalances (reflecting ceria reduction) and induction periods (reflecting active site creation) in anaerobic experiments point to ethanol dehydrogenation turnovers owing their provenance to surface reduction. Implausibly high vacancy densities obtained from analysis of oxygen imbalances when using acetaldehyde and CO2 formation rates, unlike those obtained when using water and CO2 formation rates, point to the catalytic origin of at least part of the acetaldehyde formed during both aerobic–anaerobic switches and anaerobic induction periods. Normalized water molar flow rates, used as a measure of the relative contributions of catalytic and stoichiometric routes to acetaldehyde formation, evince a transition from stoichiometric ethanol oxidation to catalytic ethanol dehydrogenation upon progressive surface reduction. High-temperature hydrogen pretreatments can be used to manipulate both the initial contribution of ethanol dehydrogenation to overall acetaldehyde formation as well as the fractional surface reduction that ethanol, rather than molecular hydrogen, effectuates. Alpha hydrogen-free titrants such as phenol, on the other hand, can be used to titrate sites contributing to catalytic alkanol dehydrogenation without altering the prevalence of stoichiometric routes responsible for site creation in the first place. The combination of transient experiments used herein capture, with clarity, the evolution in catalytic function of high-surface-area cerium oxide toward ethanol upon progressive reduction that originates from its stoichiometric conversion over a native, fully oxidized surface. Not unimportantly, the results also point to an avenue for the water-free synthesis of alkanals over reducible metal oxides.
中文翻译:

还原性金属氧化物向催化非氧化链烷醇脱氢反应过程的瞬态动力学分析
对四组需氧/厌氧乙醇转化瞬态的定量分析表明,天然高表面积氧化铈表面的演化会影响乙醇氧化转化率的一半降低,从而仅在完全还原表面后催化非氧化乙醇脱氢。498 K的有氧-厌氧转换导致新的稳态脱氢速率,而不是催化乙醛形成的终止。在厌氧实验中,氧失衡(反映氧化铈还原)和诱导期(反映活性位点生成)的同时终止表明乙醇脱氢转化率是由于其表面还原引起的。通过使用乙醛和CO 2时的氧气失衡分析获得的空位密度高得令人难以置信形成速率,与使用水和CO 2时获得的速率不同形成速率,指向有氧-厌氧转换和厌氧诱导期间形成的至少一部分乙醛的催化起源。归一化的水摩尔流量,用作催化和化学计量路线对乙醛形成的相对贡献的量度,避免了在逐步进行表面还原时从化学计量乙醇氧化转变为催化乙醇脱氢的过程。高温氢预处理可用于控制乙醇脱氢对整个乙醛形成的最初贡献以及乙醇而不是分子氢实现的部分表面还原。另一方面,无氢滴定剂,例如苯酚,可用于滴定有助于催化链烷醇脱氢的位点,而不会首先改变负责位点形成的化学计量路线的发生率。本文使用的瞬态实验的结合清楚地捕获了高表面积氧化铈在逐步还原时朝乙醇的催化功能的演变,这是由其在天然的,完全氧化的表面上的化学计量转化引起的。同样重要的是,该结果还指出了在可还原金属氧化物上无水合成链烷烃的途径。逐渐还原的过程中,高表面积氧化铈对乙醇的催化功能演变,这是由于其在天然,完全氧化的表面上的化学计量转化所致。同样重要的是,该结果还指出了在可还原金属氧化物上无水合成链烷烃的途径。逐渐还原的过程中,高表面积氧化铈对乙醇的催化功能演变,这是由于其在天然,完全氧化的表面上的化学计量转化所致。同样重要的是,该结果还指出了在可还原金属氧化物上无水合成链烷烃的途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号