Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2020-08-28 , DOI: 10.1016/j.bioorg.2020.104168 Abida Munir 1 , Adil Khushal 1 , Kiran Saeed 1 , Abdul Sadiq 2 , Rahim Ullah 3 , Gowhar Ali 3 , Zaman Ashraf 4 , Ehsan Ullah Mughal 5 , Muhammad Saeed Jan 2 , Umer Rashid 1 , Izhar Hussain 6 , Amara Mumtaz 1
|
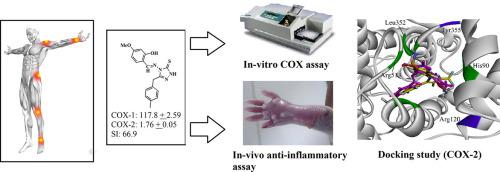
Over the course of time several drugs have been synthesized and are available in market for the treatment of inflammation. However, they were unable to cure effectively and associated with side effects. To effectively deal with such diseases, heterocycles and their derivatives have gained their special position. For this reason 1,3,4-oxadiazole (15–16), 1,2,4-triazole (17–18), Schiff base (19–24) and 3,5-disubstituted pyrazole (25) derivatives were synthesized starting from salicylic acid and acyl acid hydrazides (12–14) as COX-1 and COX-2 inhibitors. In vivo anti-inflammatory activities were also tested by carrageenan-induced mice paw edema against albino mice of any sex. Structures of all the synthesized compounds were confirmed by FT-IR and 1H NMR analysis. Schiff base derivative of 4-amiontirazole (24) with IC50 value of 1.76 ± 0.05 (COX-2) and 117.8 ± 2.59 emerged as potent COX-2 inhibitor. Furthermore, we also performed in-vivo anti-inflammatory investigations by using carrageenan induced paw edema test. From in-vivo anti-inflammatory activities, it was found that after 1 h the maximum percentage inhibition 15.8% was observed by compound 14 which is comparable with that of the standard drug followed by the compound 18 with percentage inhibition of 10.5%. After 3 h, the maximum percentage inhibition was observed by compound 18 with 22.2% and compound 14 with 16.7%. After 5 h the maximum percentage inhibition was observed by compound 18 with 29.4% followed by compound 16 with 23.5%. We further explore the mechanism of the inhibition by using docking simulations. Docking studies revealed that the selective COX-2 inhibitors established interactions with additional COX-2 enzyme pocket residues.
中文翻译:

酰基和水杨酸酰肼衍生物的合成,体外,体内抗炎活性和分子对接研究。
随着时间的流逝,已经合成了几种药物,并且在市场上可用于治疗炎症。但是,他们无法有效治愈并伴有副作用。为了有效地应对此类疾病,杂环及其衍生物已获得特殊地位。因此,首先合成了1,3,4-恶二唑(15 – 16),1,2,4-三唑(17 – 18),席夫碱(19 – 24)和3,5-二取代吡唑(25)衍生物。由水杨酸和酰肼(12 – 14)用作COX-1和COX-2抑制剂。体内还通过角叉菜胶诱导的小鼠爪水肿针对任何性别的白化病小鼠测试了抗炎活性。通过FT-IR和1 H NMR分析确认所有合成化合物的结构。出现了4-氨基噻唑(24)的席夫碱衍生物,IC 50值为1.76±0.05(COX-2)和117.8±2.59,作为有效的COX-2抑制剂。此外,我们还通过使用角叉菜胶诱导的爪水肿试验进行了体内抗炎研究。从在-体内抗炎活性,发现,1个小时的最大抑制百分比后,通过化合物观察到15.8%14与标准药物相当,随后是化合物18,其抑制百分比为10.5%。3小时后,观察到最大的抑制百分率是化合物18为22.2%,化合物14为16.7%。5小时后,观察到最大的抑制百分率是化合物18(29.4%),然后化合物16(23.5%)。我们通过使用对接模拟进一步探索抑制的机制。对接研究表明,选择性COX-2抑制剂与其他COX-2酶口袋残基建立了相互作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号