当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A removable directing group-assisted Rh(III)-catalyzed direct C–H bond activation/annulation cascade to synthesize highly fused isoquinolines
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2020-08-27 , DOI: 10.1039/d0qo00786b Yilang Cheng 1, 2, 3, 4, 5 , Xu Han 1, 2, 3, 4, 5 , Junyou Li 1, 2, 3, 4, 5 , Yu Zhou 1, 2, 3, 4, 5 , Hong Liu 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2020-08-27 , DOI: 10.1039/d0qo00786b Yilang Cheng 1, 2, 3, 4, 5 , Xu Han 1, 2, 3, 4, 5 , Junyou Li 1, 2, 3, 4, 5 , Yu Zhou 1, 2, 3, 4, 5 , Hong Liu 1, 2, 3, 4, 5
Affiliation
|
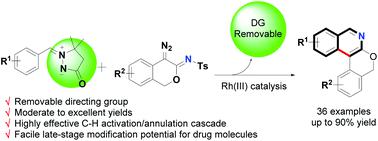
An azomethine imine-directed Rh(III)-catalyzed C–H functionalization/annulation cascade to construct highly fused isoquinoline scaffolds, in which the azomethine imine was used as a removable directing group and could be removed in situ under mild reaction conditions with good to excellent yields, accompanied by a broad substrate scope and good functional group tolerance was developed. Notably, this strategy could be further extended to late-stage modification of drug molecules, offering more potential in drug discoveries.
中文翻译:

可移动的导向基团辅助的Rh(III)催化的直接C–H键活化/环化级联反应,以合成高度稠合的异喹啉
甲亚胺亚胺定向的Rh(III)催化的C–H功能化/环构级联反应,以构建高度融合的异喹啉支架,其中将甲亚胺亚胺用作可移动的导向基团,可以在温和的反应条件下原位去除,具有良好的开发出了优异的产率,并伴随着广泛的底物范围和良好的官能团耐受性。值得注意的是,该策略可以进一步扩展到药物分子的后期修饰,在药物发现中提供更大的潜力。
更新日期:2020-10-13
中文翻译:

可移动的导向基团辅助的Rh(III)催化的直接C–H键活化/环化级联反应,以合成高度稠合的异喹啉
甲亚胺亚胺定向的Rh(III)催化的C–H功能化/环构级联反应,以构建高度融合的异喹啉支架,其中将甲亚胺亚胺用作可移动的导向基团,可以在温和的反应条件下原位去除,具有良好的开发出了优异的产率,并伴随着广泛的底物范围和良好的官能团耐受性。值得注意的是,该策略可以进一步扩展到药物分子的后期修饰,在药物发现中提供更大的潜力。










































 京公网安备 11010802027423号
京公网安备 11010802027423号