当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Using reverse osmosis membranes to control ion transport during water electrolysis
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2020-08-26 , DOI: 10.1039/d0ee02173c Le Shi 1, 2, 3, 4 , Ruggero Rossi 1, 2, 3, 4 , Moon Son 1, 2, 3, 4 , Derek M. Hall 2, 3, 4, 5 , Michael A. Hickner 2, 3, 4, 6 , Christopher A. Gorski 1, 2, 3, 4 , Bruce E. Logan 1, 2, 3, 4
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2020-08-26 , DOI: 10.1039/d0ee02173c Le Shi 1, 2, 3, 4 , Ruggero Rossi 1, 2, 3, 4 , Moon Son 1, 2, 3, 4 , Derek M. Hall 2, 3, 4, 5 , Michael A. Hickner 2, 3, 4, 6 , Christopher A. Gorski 1, 2, 3, 4 , Bruce E. Logan 1, 2, 3, 4
Affiliation
|
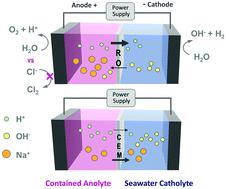
The decreasing cost of electricity produced using solar and wind and the need to avoid CO2 emissions from fossil fuels has heightened interest in hydrogen gas production by water electrolysis. Offshore and coastal hydrogen gas production using seawater and renewable electricity is of particular interest, but it is currently economically infeasible due to the high costs of ion exchange membranes and the need to desalinate seawater in existing electrolyzer designs. A new approach is described here that uses relatively inexpensive commercially available membranes developed for reverse osmosis (RO) to selectively transport favorable ions. In an applied electric field, RO membranes have a substantial capacity for proton and hydroxide transport through the active layer while excluding salt anions and cations. A perchlorate salt was used to provide an inert and contained anolyte, with charge balanced by proton and hydroxide ion flow across the RO membrane. Synthetic seawater (NaCl) was used as the catholyte, where it provided continuous hydrogen gas evolution. The RO membrane resistance was 21.7 ± 3.5 Ω cm2 in 1 M NaCl and the voltages needed to split water in a model electrolysis cell at current densities of 10–40 mA cm−2 were comparable to those found when using two commonly used, more expensive ion exchange membranes.
中文翻译:

在水电解过程中使用反渗透膜控制离子传输
使用太阳能和风能发电的成本不断降低,并且需要避免CO 2化石燃料的排放已引起人们对通过水电解生产氢气的兴趣。使用海水和可再生电力生产近海和沿海氢气特别令人关注,但是由于离子交换膜的高成本以及在现有电解槽设计中需要对海水进行脱盐处理,目前在经济上是不可行的。本文介绍了一种新方法,该方法使用为反渗透(RO)开发的相对便宜的市售膜来选择性地传输有利离子。在外加电场中,RO膜具有大量的质子和氢氧化物通过活性层传输的能力,同时不包含盐阴离子和阳离子。高氯酸盐用来提供惰性的阳极电解液,电荷由质子和氢氧根离子流过RO膜平衡。合成海水(NaCl)用作阴极电解液,可提供连续的氢气逸出。反渗透膜电阻为21.7±3.5Ωcm2 in 1 M NaCl和在模型电解槽中以10–40 mA cm -2的电流密度分解水所需的电压与使用两个常用的,更昂贵的离子交换膜时的电压相当。
更新日期:2020-09-16
中文翻译:

在水电解过程中使用反渗透膜控制离子传输
使用太阳能和风能发电的成本不断降低,并且需要避免CO 2化石燃料的排放已引起人们对通过水电解生产氢气的兴趣。使用海水和可再生电力生产近海和沿海氢气特别令人关注,但是由于离子交换膜的高成本以及在现有电解槽设计中需要对海水进行脱盐处理,目前在经济上是不可行的。本文介绍了一种新方法,该方法使用为反渗透(RO)开发的相对便宜的市售膜来选择性地传输有利离子。在外加电场中,RO膜具有大量的质子和氢氧化物通过活性层传输的能力,同时不包含盐阴离子和阳离子。高氯酸盐用来提供惰性的阳极电解液,电荷由质子和氢氧根离子流过RO膜平衡。合成海水(NaCl)用作阴极电解液,可提供连续的氢气逸出。反渗透膜电阻为21.7±3.5Ωcm2 in 1 M NaCl和在模型电解槽中以10–40 mA cm -2的电流密度分解水所需的电压与使用两个常用的,更昂贵的离子交换膜时的电压相当。







































 京公网安备 11010802027423号
京公网安备 11010802027423号