当前位置:
X-MOL 学术
›
Biomater. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A frog-derived bionic peptide with discriminative inhibition of tumors based on integrin αvβ3 identification.
Biomaterials Science ( IF 5.8 ) Pub Date : 2020-08-27 , DOI: 10.1039/d0bm01187h Zhihao Han 1 , Chen Lian , Yuxuan Ma , Congying Zhang , Zicun Liu , Yuanbiao Tu , Yi Ma , Yueqing Gu
Biomaterials Science ( IF 5.8 ) Pub Date : 2020-08-27 , DOI: 10.1039/d0bm01187h Zhihao Han 1 , Chen Lian , Yuxuan Ma , Congying Zhang , Zicun Liu , Yuanbiao Tu , Yi Ma , Yueqing Gu
Affiliation
|
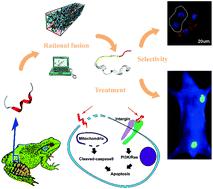
Aureins, natural active peptides extracted from skin secretions of Australian bell frogs, have become a research focus due to the antitumor effects caused by lysing cell membranes. However, clinical translation of Aureins is still limited by non-selective toxicity between normal and cancer cells. Herein, by structure–activity relationship analysis and rational linker design, a dual-function fusion peptide RA3 is designed by tactically fusing Aurein peptide A1 with strong anticancer activity, with a tri-peptide with integrin αvβ3-binding ability which was screened in our previous work. Rational design and selection of fusion linkers ensures α-helical conformation and active functions of this novel fusion peptide, inducing effective membrane rupture and selective apoptosis of cancer cells. The integrin binding and tumor recognition ability of the fusion peptide is further validated by fluorescence imaging in cell and mouse models, in comparison with the non-selective A1 peptide. Meanwhile, increased stability and superior therapeutic efficacy are achieved in vivo for the RA3 fusion peptide. Our study highlights that aided by computational simulation technologies, the biomimetic fusion RA3 peptide has been successfully designed, surmounting the poor tumor-selectivity of the natural defensive peptide, serving as a promising therapeutic agent for cancer treatment.
中文翻译:

基于整联蛋白αvβ3鉴定的具有歧视性抑制性的青蛙衍生仿生肽。
由于澳大利亚细胞膜蛙的皮肤分泌物具有抗肿瘤作用,从澳大利亚钟蛙的皮肤分泌物中提取的天然活性肽金黄色素已成为研究的重点。然而,Aureins的临床翻译仍然受到正常细胞与癌细胞之间非选择性毒性的限制。在本文中,通过结构-活性关系分析和合理的接头设计,通过战术融合具有强抗癌活性的金黄色素肽A1和具有整合素αvβ3结合能力的三肽,设计了双功能融合肽RA3。工作。融合接头的合理设计和选择可确保这种新型融合肽的α螺旋构象和活性功能,从而诱导有效的膜破裂和癌细胞的选择性凋亡。与非选择性A1肽相比,融合肽的整联蛋白结合和肿瘤识别能力在细胞和小鼠模型中通过荧光成像得到了进一步验证。同时,实现了更高的稳定性和出色的治疗功效RA3融合肽在体内。我们的研究表明,借助计算机模拟技术,仿生融合RA3肽已成功设计,克服了天然防御肽对肿瘤的选择性差的缺点,成为有希望的癌症治疗剂。
更新日期:2020-09-22
中文翻译:

基于整联蛋白αvβ3鉴定的具有歧视性抑制性的青蛙衍生仿生肽。
由于澳大利亚细胞膜蛙的皮肤分泌物具有抗肿瘤作用,从澳大利亚钟蛙的皮肤分泌物中提取的天然活性肽金黄色素已成为研究的重点。然而,Aureins的临床翻译仍然受到正常细胞与癌细胞之间非选择性毒性的限制。在本文中,通过结构-活性关系分析和合理的接头设计,通过战术融合具有强抗癌活性的金黄色素肽A1和具有整合素αvβ3结合能力的三肽,设计了双功能融合肽RA3。工作。融合接头的合理设计和选择可确保这种新型融合肽的α螺旋构象和活性功能,从而诱导有效的膜破裂和癌细胞的选择性凋亡。与非选择性A1肽相比,融合肽的整联蛋白结合和肿瘤识别能力在细胞和小鼠模型中通过荧光成像得到了进一步验证。同时,实现了更高的稳定性和出色的治疗功效RA3融合肽在体内。我们的研究表明,借助计算机模拟技术,仿生融合RA3肽已成功设计,克服了天然防御肽对肿瘤的选择性差的缺点,成为有希望的癌症治疗剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号