当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Accessing the Activation Mechanisms of Ethylene Photo-Polymerization under Pressure by Transient Infrared Absorption Spectroscopy.
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-08-26 , DOI: 10.1021/acs.jpcb.0c06244 Sebastiano Romi 1 , Samuele Fanetti 1, 2 , Roberto Bini 1, 2, 3
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-08-26 , DOI: 10.1021/acs.jpcb.0c06244 Sebastiano Romi 1 , Samuele Fanetti 1, 2 , Roberto Bini 1, 2, 3
Affiliation
|
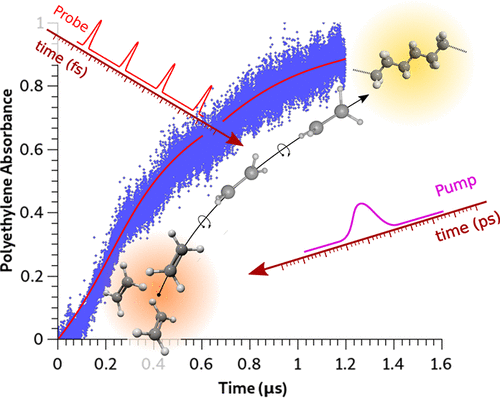
The ambient temperature photoinduced polymerization of compressed (P < 1 GPa) fluid ethylene was characterized by transient infrared absorption spectroscopy with a resolution of few nanoseconds, 3 orders of magnitude higher than previously reported. The reaction has been studied under both one- and two-photon excitation evidencing in the latter case its occurrence only in the presence of different transition metal oxides. Their photocatalytic activity is ascribed to the stabilization of the excited biradicals through electron density exchange between the d orbitals of the metal and the π antibonding orbitals of ethylene which lengthens the lifetime of the biradicals. In both one- and two-photon activation cases the polymerization is characterized by an initial step distinguished by a molecularity of 0.15 ± 0.02 identified as the activation step of the reaction lasting, in the one-photon excitation case, a few hundreds of nanoseconds. Using pulsed excitation the reaction evolves toward a free radical polymerization only under one-photon excitation whereas the critical concentration of radicals required to propagate the reaction is never achieved in the two-photon excitation case. Comparison with continuous wave excitation unambiguously identifies in the average power released to the sample the key factor to drive quantitatively and qualitatively the polymerization.
中文翻译:

通过瞬态红外吸收光谱法研究压力下乙烯光聚合的活化机理。
常温下光致聚合压缩(P小于1 GPa的液态乙烯通过瞬态红外吸收光谱法进行表征,其分辨率为几纳秒,比以前报道的结果高3个数量级。已经在单光子激发和双光子激发下研究了该反应,证明在后一种情况下仅在存在不同的过渡金属氧化物的情况下才发生该反应。它们的光催化活性归因于通过在金属的d轨道与乙烯的π反键轨道之间进行电子密度交换来激发双自由基的稳定性,从而延长了双自由基的寿命。在单光子和双光子活化情况下,聚合反应均以初始步骤为特征,该步骤的特征在于分子的0.15±0.02被确定为在单光子激发情况下持续进行的反应活化步骤。几百纳秒。使用脉冲激发,仅在单光子激发下,反应发展为自由基聚合,而在双光子激发的情况下,传播该反应所需的自由基的临界浓度从未达到。与连续波激发的比较清楚地确定了释放到样品中的平均功率,这是定量和定性驱动聚合反应的关键因素。
更新日期:2020-09-18
中文翻译:

通过瞬态红外吸收光谱法研究压力下乙烯光聚合的活化机理。
常温下光致聚合压缩(P小于1 GPa的液态乙烯通过瞬态红外吸收光谱法进行表征,其分辨率为几纳秒,比以前报道的结果高3个数量级。已经在单光子激发和双光子激发下研究了该反应,证明在后一种情况下仅在存在不同的过渡金属氧化物的情况下才发生该反应。它们的光催化活性归因于通过在金属的d轨道与乙烯的π反键轨道之间进行电子密度交换来激发双自由基的稳定性,从而延长了双自由基的寿命。在单光子和双光子活化情况下,聚合反应均以初始步骤为特征,该步骤的特征在于分子的0.15±0.02被确定为在单光子激发情况下持续进行的反应活化步骤。几百纳秒。使用脉冲激发,仅在单光子激发下,反应发展为自由基聚合,而在双光子激发的情况下,传播该反应所需的自由基的临界浓度从未达到。与连续波激发的比较清楚地确定了释放到样品中的平均功率,这是定量和定性驱动聚合反应的关键因素。











































 京公网安备 11010802027423号
京公网安备 11010802027423号