当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development of Novel AKR1C3 Inhibitors as New Potential Treatment for Castration-Resistant Prostate Cancer.
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2020-08-27 , DOI: 10.1021/acs.jmedchem.0c00939 Satoshi Endo 1 , Hiroaki Oguri 1 , Jin Segawa 1 , Mina Kawai 1 , Dawei Hu 2 , Shuang Xia 2 , Takuya Okada 2 , Katsumasa Irie 3, 4 , Shinya Fujii 5 , Hiroaki Gouda 6 , Kazuhiro Iguchi 7 , Takuo Matsukawa 8 , Naohiro Fujimoto 8 , Toshiyuki Nakayama 9 , Naoki Toyooka 2 , Toshiyuki Matsunaga 10 , Akira Ikari 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2020-08-27 , DOI: 10.1021/acs.jmedchem.0c00939 Satoshi Endo 1 , Hiroaki Oguri 1 , Jin Segawa 1 , Mina Kawai 1 , Dawei Hu 2 , Shuang Xia 2 , Takuya Okada 2 , Katsumasa Irie 3, 4 , Shinya Fujii 5 , Hiroaki Gouda 6 , Kazuhiro Iguchi 7 , Takuo Matsukawa 8 , Naohiro Fujimoto 8 , Toshiyuki Nakayama 9 , Naoki Toyooka 2 , Toshiyuki Matsunaga 10 , Akira Ikari 1
Affiliation
|
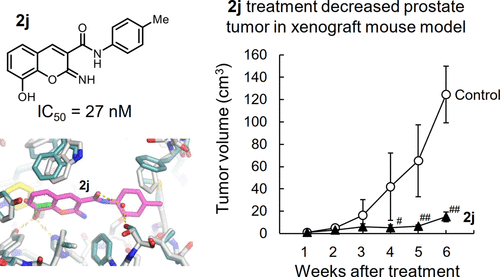
Aldo–keto reductase (AKR) 1C3 catalyzes the synthesis of active androgens that promote the progression of prostate cancer. AKR1C3 also contributes to androgen-independent cell proliferation and survival through the metabolism of prostaglandins and reactive aldehydes. Because of its elevation in castration-resistant prostate cancer (CRPC) tissues, AKR1C3 is a promising therapeutic target for CRPC. In this study, we found a novel potent AKR1C3 inhibitor, N-(4-fluorophenyl)-8-hydroxy-2-imino-2H-chromene-3-carboxamide (2d), and synthesized its derivatives with IC50 values of 25–56 nM and >220-fold selectivity over other AKRs (1C1, 1C2, and 1C4). The structural factors for the inhibitory potency were elucidated by crystallographic study of AKR1C3 complexes with 2j and 2l. The inhibitors suppressed proliferation of prostate cancer 22Rv1 and PC3 cells through both androgen-dependent and androgen-independent mechanisms. Additionally, 2j and 2l prevented prostate tumor growth in a xenograft mouse model. Furthermore, the inhibitors significantly augmented apoptotic cell death induced by anti-CRPC drugs (abiraterone or enzalutamide).
中文翻译:

新型AKR1C3抑制剂的开发作为去势抵抗性前列腺癌的新潜在治疗方法。
醛基酮还原酶(AKR)1C3催化活性雄激素的合成,从而促进前列腺癌的发展。AKR1C3还通过前列腺素和反应性醛的代谢促进非雄激素依赖性细胞增殖和存活。由于其在去势抵抗性前列腺癌(CRPC)组织中的升高,AKR1C3是CRPC的有希望的治疗靶标。在这项研究中,我们发现了一种新型的强效AKR1C3抑制剂N-(4-氟苯基)-8-羟基-2-亚氨基-2 H-色烯-3-羧酰胺(2d),并用IC 50合成了其衍生物值比其他AKR(1C1、1C2和1C4)高25-56 nM,选择性> 220倍。通过结晶研究AKR1C3与2j和2l的复合物阐明了抑制效力的结构因素。所述抑制剂通过雄激素依赖性和雄激素依赖性机制抑制前列腺癌22Rv1和PC3细胞的增殖。另外,2j和2l阻止了异种移植小鼠模型中前列腺肿瘤的生长。此外,这些抑制剂显着增加了由抗CRPC药物(阿比特龙或enzalutamide)诱导的凋亡细胞死亡。
更新日期:2020-09-24
中文翻译:

新型AKR1C3抑制剂的开发作为去势抵抗性前列腺癌的新潜在治疗方法。
醛基酮还原酶(AKR)1C3催化活性雄激素的合成,从而促进前列腺癌的发展。AKR1C3还通过前列腺素和反应性醛的代谢促进非雄激素依赖性细胞增殖和存活。由于其在去势抵抗性前列腺癌(CRPC)组织中的升高,AKR1C3是CRPC的有希望的治疗靶标。在这项研究中,我们发现了一种新型的强效AKR1C3抑制剂N-(4-氟苯基)-8-羟基-2-亚氨基-2 H-色烯-3-羧酰胺(2d),并用IC 50合成了其衍生物值比其他AKR(1C1、1C2和1C4)高25-56 nM,选择性> 220倍。通过结晶研究AKR1C3与2j和2l的复合物阐明了抑制效力的结构因素。所述抑制剂通过雄激素依赖性和雄激素依赖性机制抑制前列腺癌22Rv1和PC3细胞的增殖。另外,2j和2l阻止了异种移植小鼠模型中前列腺肿瘤的生长。此外,这些抑制剂显着增加了由抗CRPC药物(阿比特龙或enzalutamide)诱导的凋亡细胞死亡。











































 京公网安备 11010802027423号
京公网安备 11010802027423号