当前位置:
X-MOL 学术
›
ACS Pharmacol. Transl. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cyclohepta[b]thiophenes as Potential Antiproliferative Agents: Design, Synthesis, In Vitro, and In Vivo Anticancer Evaluation
ACS Pharmacology & Translational Science ( IF 4.9 ) Pub Date : 2020-08-27 , DOI: 10.1021/acsptsci.0c00096 Somaya A Abdel-Rahman 1, 2 , Ashraf K El-Damasy 2 , Ghada S Hassan 2 , Emad I Wafa 1 , Sean M Geary 1 , Azza R Maarouf 2 , Aliasger K Salem 1
ACS Pharmacology & Translational Science ( IF 4.9 ) Pub Date : 2020-08-27 , DOI: 10.1021/acsptsci.0c00096 Somaya A Abdel-Rahman 1, 2 , Ashraf K El-Damasy 2 , Ghada S Hassan 2 , Emad I Wafa 1 , Sean M Geary 1 , Azza R Maarouf 2 , Aliasger K Salem 1
Affiliation
|
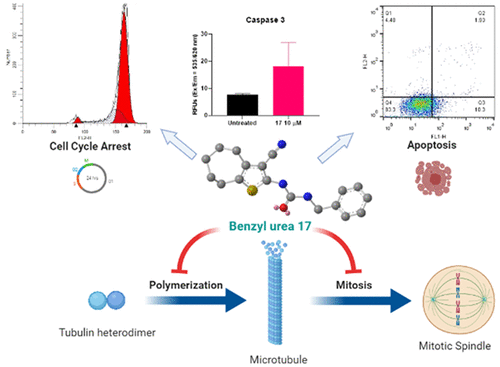
Several thiophene featuring compounds are known for their promising antiproliferative activity. Prompted by the urgent need to identify new potent anticancer agents, 16 compounds of benzamides, benzylamines, and urea analogues incorporating a cyclohepta[b]thiophene scaffold were synthesized and biologically evaluated with a cell proliferation assay using the A549 nonsmall cell lung cancer cell line. Compound 17 demonstrated both potent and broad-spectrum anticancer activity with submicromolar 50% growth inhibition (GI50) values. It also showed superior antiproliferative activity (vs nocodazole) in OVACAR-4, OVACAR-5, CAKI-1, and T47D cell lines with GI50 values of 2.01 (vs 22.28), 2.27 (vs 20.75), 0.69 (vs 1.11), and 0.362 (vs 81.283) μM, respectively. Additionally, compound 17 displayed minimal cytotoxicity based on 50% lethal concentration (LC50) values toward all tested cell lines. Further cell-based mechanistic studies of compound 17 revealed its ability to induce cell cycle arrest of A549 cells as evidenced by dose dependent G2/M accumulation. Furthermore, induction of early apoptosis along with activation of caspase 3, 8, and 9 were confirmed in A549 cells treated with compound 17. Targeting tubulin polymerization may explain the mechanism of the antiproliferative activity of compound 17 based on cell cycle analysis, detected apoptosis, and in vitro inhibition of tubulin polymerization. In vitro data were further supported by in vivo antitumor efficacy studies of compound 17 in a CT26 murine model for which the results showed a reduction in the tumor growth compared to untreated mice. Overall, compound 17 has the potential to function as a promising candidate for further development of potent anticancer chemotherapeutics.
中文翻译:

环庚[b]噻吩作为潜在的抗增殖剂:设计、合成、体外和体内抗癌评估
几种以噻吩为特征的化合物以其有希望的抗增殖活性而闻名。由于迫切需要鉴定新的强效抗癌剂,合成了 16 种苯甲酰胺、苯甲胺和尿素类似物的化合物,其中结合了环庚[ b ]噻吩支架,并使用 A549 非小细胞肺癌细胞系通过细胞增殖试验进行了生物学评估。化合物17表现出强效和广谱的抗癌活性,具有亚微摩尔 50% 的生长抑制 (GI 50 ) 值。它还在具有 GI 50的 OVACAR-4、OVACAR-5、CAKI-1 和 T47D 细胞系中显示出优异的抗增殖活性(与诺考达唑相比)值分别为 2.01 (vs 22.28)、2.27 (vs 20.75)、0.69 (vs 1.11) 和 0.362 (vs 81.283) μM。此外,基于 50% 致死浓度 (LC 50 ) 值,化合物17对所有测试的细胞系显示出最小的细胞毒性。化合物17的进一步基于细胞的机制研究揭示了其诱导 A549 细胞细胞周期停滞的能力,如剂量依赖性 G 2 /M 积累所证明的那样。此外,在用化合物17处理的 A549 细胞中证实了早期细胞凋亡的诱导以及半胱天冬酶 3、8 和 9 的激活。靶向微管蛋白聚合可以解释化合物17的抗增殖活性机制基于细胞周期分析,检测到细胞凋亡和体外抑制微管蛋白聚合。化合物17在 CT26 鼠模型中的体内抗肿瘤功效研究进一步支持了体外数据,结果显示与未治疗的小鼠相比,肿瘤生长减少。总体而言,化合物17有可能成为进一步开发有效的抗癌化疗药物的有希望的候选药物。
更新日期:2020-10-11
中文翻译:

环庚[b]噻吩作为潜在的抗增殖剂:设计、合成、体外和体内抗癌评估
几种以噻吩为特征的化合物以其有希望的抗增殖活性而闻名。由于迫切需要鉴定新的强效抗癌剂,合成了 16 种苯甲酰胺、苯甲胺和尿素类似物的化合物,其中结合了环庚[ b ]噻吩支架,并使用 A549 非小细胞肺癌细胞系通过细胞增殖试验进行了生物学评估。化合物17表现出强效和广谱的抗癌活性,具有亚微摩尔 50% 的生长抑制 (GI 50 ) 值。它还在具有 GI 50的 OVACAR-4、OVACAR-5、CAKI-1 和 T47D 细胞系中显示出优异的抗增殖活性(与诺考达唑相比)值分别为 2.01 (vs 22.28)、2.27 (vs 20.75)、0.69 (vs 1.11) 和 0.362 (vs 81.283) μM。此外,基于 50% 致死浓度 (LC 50 ) 值,化合物17对所有测试的细胞系显示出最小的细胞毒性。化合物17的进一步基于细胞的机制研究揭示了其诱导 A549 细胞细胞周期停滞的能力,如剂量依赖性 G 2 /M 积累所证明的那样。此外,在用化合物17处理的 A549 细胞中证实了早期细胞凋亡的诱导以及半胱天冬酶 3、8 和 9 的激活。靶向微管蛋白聚合可以解释化合物17的抗增殖活性机制基于细胞周期分析,检测到细胞凋亡和体外抑制微管蛋白聚合。化合物17在 CT26 鼠模型中的体内抗肿瘤功效研究进一步支持了体外数据,结果显示与未治疗的小鼠相比,肿瘤生长减少。总体而言,化合物17有可能成为进一步开发有效的抗癌化疗药物的有希望的候选药物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号