当前位置:
X-MOL 学术
›
J. Neurochem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A low amyloidogenic E61K transthyretin mutation may cause familial amyloid polyneuropathy
Journal of Neurochemistry ( IF 4.2 ) Pub Date : 2020-08-27 , DOI: 10.1111/jnc.15162 Tatsufumi Murakami 1 , Takeshi Yokoyama 2 , Mineyuki Mizuguchi 2 , Shigenobu Toné 3 , Shizuka Takaku 4 , Kazunori Sango 4 , Hirotake Nishimura 5 , Kazuhiko Watabe 6 , Yoshihide Sunada 1
Journal of Neurochemistry ( IF 4.2 ) Pub Date : 2020-08-27 , DOI: 10.1111/jnc.15162 Tatsufumi Murakami 1 , Takeshi Yokoyama 2 , Mineyuki Mizuguchi 2 , Shigenobu Toné 3 , Shizuka Takaku 4 , Kazunori Sango 4 , Hirotake Nishimura 5 , Kazuhiko Watabe 6 , Yoshihide Sunada 1
Affiliation
|
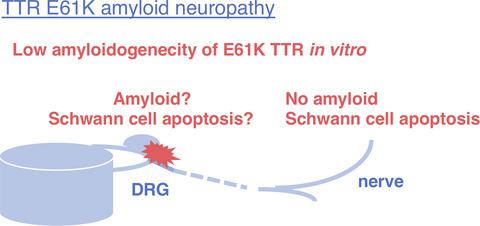
Patients with transthyretin (TTR)‐type familial amyloid polyneuropathy (FAP) typically exhibit sensory dominant polyneuropathy and autonomic neuropathy. However, the molecular pathogenesis of the neuropathy remains unclear. In this study, we characterize the features of FAP TTR the substitution of lysine for glutamic acid at position 61 (E61K). This FAP was late‐onset, with sensory dominant polyneuropathy, autonomic neuropathy, and cardiac amyloidosis. Interestingly, no amyloid deposits were found in the endoneurium of the four nerve specimens examined. Therefore, we examined the amyloidogenic properties of E61K TTR in vitro. Recombinant wild‐type TTR, the substitution of methionine for valine at position 30 (V30M) TTR, and E61K TTR proteins were incubated at 37°C for 72 hr, and amyloid fibril formation was assessed using the thioflavin‐T binding assay. Amyloid fibril formation by E61K TTR was less than that by V30M TTR, and similar to that by wild‐type TTR. E61K TTR did not have an inhibitory effect on neurite outgrowth from adult rat dorsal root ganglion (DRG) neurons, but V30M TTR did. Furthermore, we studied the sural nerve of our patient by terminal deoxynucleotidyl transferase dUTP nick end labeling and electron microscopy. A number of apoptotic cells were observed in the endoneurium of the nerve by transferase dUTP nick end labeling. Chromatin condensation was confirmed in the nucleus of non‐myelinating Schwann cells by electron microscopy. These findings suggest that E61K TTR is low amyloidogenic, in vitro and in vivo. However, TTR aggregates and amyloid fibrils in the DRG may cause sensory impairments in FAP because the DRG has no blood–nerve barrier. Moreover, Schwann cell apoptosis may contribute to the neurodegeneration.
中文翻译:

淀粉样蛋白低的E61K转甲状腺素蛋白突变可能导致家族性淀粉样蛋白多神经病
甲状腺素(TTR)型家族性淀粉样蛋白多发性神经病(FAP)的患者通常表现为感觉性优势多发性神经病和自主神经病。然而,神经病的分子发病机制仍不清楚。在这项研究中,我们表征了FAP TTR在61位(E61K)处用赖氨酸替代谷氨酸的特征。该FAP起病较晚,伴有感觉性优势多发性神经病,自主神经病和心脏淀粉样变性。有趣的是,在所检查的四个神经标本的神经内膜中未发现淀粉样蛋白沉积。因此,我们在体外检查了E61K TTR的淀粉样蛋白生成特性。重组野生型TTR,在30位(V30M)TTR处的蛋氨酸取代缬氨酸和E61K TTR蛋白在37°C孵育72小时,并使用thioflavin‐T结合测定法评估淀粉样蛋白原纤维的形成。E61K TTR的淀粉样原纤维形成少于V30M TTR,与野生型TTR相似。E61K TTR对成年大鼠背根神经节(DRG)神经元的神经突生长没有抑制作用,而V30M TTR有抑制作用。此外,我们通过末端脱氧核苷酸转移酶dUTP缺口末端标记和电子显微镜研究了患者的腓肠神经。通过转移酶dUTP缺口末端标记在神经的神经内膜中观察到许多凋亡细胞。通过电子显微镜证实染色质在非髓鞘雪旺细胞核中凝集。这些发现表明,E61K TTR在体外和体内均具有低淀粉样生成性。然而,DRG中的TTR聚集体和淀粉样蛋白原纤维可能会导致FAP的感觉障碍,因为DRG没有血液神经屏障。此外,雪旺氏细胞凋亡可能促进神经变性。
更新日期:2020-08-27
中文翻译:

淀粉样蛋白低的E61K转甲状腺素蛋白突变可能导致家族性淀粉样蛋白多神经病
甲状腺素(TTR)型家族性淀粉样蛋白多发性神经病(FAP)的患者通常表现为感觉性优势多发性神经病和自主神经病。然而,神经病的分子发病机制仍不清楚。在这项研究中,我们表征了FAP TTR在61位(E61K)处用赖氨酸替代谷氨酸的特征。该FAP起病较晚,伴有感觉性优势多发性神经病,自主神经病和心脏淀粉样变性。有趣的是,在所检查的四个神经标本的神经内膜中未发现淀粉样蛋白沉积。因此,我们在体外检查了E61K TTR的淀粉样蛋白生成特性。重组野生型TTR,在30位(V30M)TTR处的蛋氨酸取代缬氨酸和E61K TTR蛋白在37°C孵育72小时,并使用thioflavin‐T结合测定法评估淀粉样蛋白原纤维的形成。E61K TTR的淀粉样原纤维形成少于V30M TTR,与野生型TTR相似。E61K TTR对成年大鼠背根神经节(DRG)神经元的神经突生长没有抑制作用,而V30M TTR有抑制作用。此外,我们通过末端脱氧核苷酸转移酶dUTP缺口末端标记和电子显微镜研究了患者的腓肠神经。通过转移酶dUTP缺口末端标记在神经的神经内膜中观察到许多凋亡细胞。通过电子显微镜证实染色质在非髓鞘雪旺细胞核中凝集。这些发现表明,E61K TTR在体外和体内均具有低淀粉样生成性。然而,DRG中的TTR聚集体和淀粉样蛋白原纤维可能会导致FAP的感觉障碍,因为DRG没有血液神经屏障。此外,雪旺氏细胞凋亡可能促进神经变性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号