当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Advances in Asymmetric Amino Acid Synthesis Enabled by Radical Chemistry
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-08-26 , DOI: 10.1002/adsc.202000753 Vladimir A. Larionov 1, 2 , Nadezhda V. Stoletova 1 , Victor I. Maleev 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-08-26 , DOI: 10.1002/adsc.202000753 Vladimir A. Larionov 1, 2 , Nadezhda V. Stoletova 1 , Victor I. Maleev 1
Affiliation
|
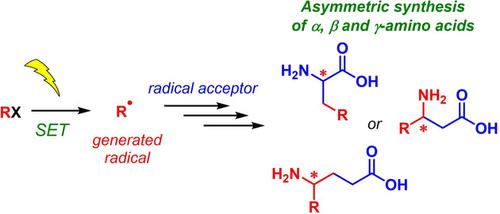
Chiral amino acids (AAs), being the main “building” blocks of the living organisms, are also an important class of organic compounds which broadly applied in synthetic chemistry, biochemistry, catalysis and the designing of new drugs. According to the industrial‐commodity market, chiral non‐proteinogenic AAs containing various functional groups come to the fore. To date, radical cross‐coupling reactions are becoming an option as an attractive powerful tool for AA syntheses. Owing to mild reaction conditions and high functional‐group tolerance, radical chemistry represents an ideal strategy for the synthesis of challenging complex non‐proteinogenic AAs. Moreover, the radical cross‐coupling allows introducing AA residue into drug scaffolds and natural compounds. In the present review, we wish to summarize and discuss all the reported to date methods of the asymmetric synthesis of AAs using radical chemistry by presenting a comprehensive account of the literature in this field going back to 1990. We especially emphasize on a radical chemistry approach and, exclusively, on stereoselective synthesis of various α‐, β‐, γ‐AAs and derivatives employing a different type of radical initiators starting from AIBN and organostannes and ending with powerful photoredox catalysis. Furthermore, the mechanism of the reported reactions will be discussed.
中文翻译:

自由基化学合成不对称氨基酸的研究进展
手性氨基酸(AAs)是生命有机体的主要“组成部分”,也是一类重要的有机化合物,广泛应用于合成化学,生物化学,催化和新药的设计。根据工业商品市场,包含各种官能团的手性非蛋白氨基酸AA脱颖而出。迄今为止,基本的交叉偶联反应已成为一种用于AA合成的有吸引力的强大工具。由于反应条件温和和对官能团的耐受性高,自由基化学代表了合成具有挑战性的复杂非蛋白氨基酸的理想方法。此外,自由基交叉偶联可将AA残基引入药物支架和天然化合物中。在目前的评论中,我们希望通过介绍可追溯到1990年的该领域文献的综述来总结和讨论迄今所有使用自由基化学方法不对称合成AA的方法。我们特别强调自由基化学方法,特别是在立体选择合成各种α-,β-,γ-AA和衍生物,它们使用不同类型的自由基引发剂,从AIBN和有机锡开始,并以强大的光氧化还原催化结束。此外,将讨论所报告反应的机理。γ-AA和衍生物采用不同类型的自由基引发剂,从AIBN和有机锡开始,并以强大的光氧化还原催化作用结束。此外,将讨论所报告反应的机理。γ-AA和衍生物使用不同类型的自由基引发剂,从AIBN和有机锡开始,并以强大的光氧化还原催化作用结束。此外,将讨论所报告反应的机理。
更新日期:2020-10-26
中文翻译:

自由基化学合成不对称氨基酸的研究进展
手性氨基酸(AAs)是生命有机体的主要“组成部分”,也是一类重要的有机化合物,广泛应用于合成化学,生物化学,催化和新药的设计。根据工业商品市场,包含各种官能团的手性非蛋白氨基酸AA脱颖而出。迄今为止,基本的交叉偶联反应已成为一种用于AA合成的有吸引力的强大工具。由于反应条件温和和对官能团的耐受性高,自由基化学代表了合成具有挑战性的复杂非蛋白氨基酸的理想方法。此外,自由基交叉偶联可将AA残基引入药物支架和天然化合物中。在目前的评论中,我们希望通过介绍可追溯到1990年的该领域文献的综述来总结和讨论迄今所有使用自由基化学方法不对称合成AA的方法。我们特别强调自由基化学方法,特别是在立体选择合成各种α-,β-,γ-AA和衍生物,它们使用不同类型的自由基引发剂,从AIBN和有机锡开始,并以强大的光氧化还原催化结束。此外,将讨论所报告反应的机理。γ-AA和衍生物采用不同类型的自由基引发剂,从AIBN和有机锡开始,并以强大的光氧化还原催化作用结束。此外,将讨论所报告反应的机理。γ-AA和衍生物使用不同类型的自由基引发剂,从AIBN和有机锡开始,并以强大的光氧化还原催化作用结束。此外,将讨论所报告反应的机理。









































 京公网安备 11010802027423号
京公网安备 11010802027423号