当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reprogramming Epoxide Hydrolase to Improve Enantioconvergence in Hydrolysis of Styrene Oxide Scaffolds
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-08-26 , DOI: 10.1002/adsc.202000898 Fu‐Long Li 1 , Yan‐Yan Qiu 1 , Yu‐Cong Zheng 1 , Fei‐Fei Chen 1 , Xu–Dong Kong 1 , Jian‐He Xu 1 , Hui‐Lei Yu 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-08-26 , DOI: 10.1002/adsc.202000898 Fu‐Long Li 1 , Yan‐Yan Qiu 1 , Yu‐Cong Zheng 1 , Fei‐Fei Chen 1 , Xu–Dong Kong 1 , Jian‐He Xu 1 , Hui‐Lei Yu 1
Affiliation
|
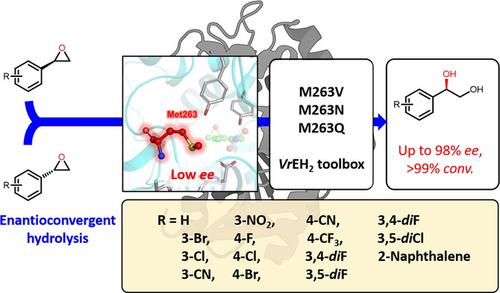
Enantioconvergent hydrolysis by epoxide hydrolase is a promising method for the synthesis of important vicinal diols. However, the poor regioselectivity of the naturally occurring enzymes results in low enantioconvergence in the enzymatic hydrolysis of styrene oxides. Herein, modulated residue No. 263 was redesigned based on structural information and a smart variant library was constructed by site‐directed modification using an “optimized amino acid alphabet” to improve the regioselectivity of epoxide hydrolase from Vigna radiata (VrEH2). The regioselectivity coefficient (r) of variant M263Q for the R‐isomer of meta‐substituted styrene oxides was improved 40–63‐fold, and variant M263V also exhibited higher regioselectivity towards the R‐isomer of para‐substituted styrene oxides compared with the wild type, which resulted in improved enantioconvergence in hydrolysis of styrene oxide scaffolds. Structural insight showed the crucial role of residue No. 263 in modulating the substrate binding conformation by altering the binding surroundings. Furthermore, increased differences in the attacking distance between nucleophilic residue Asp101 and the two carbon atoms of the epoxide ring provided evidence for improved regioselectivity. Several high‐value vicinal diols were readily synthesized (>88% yield, 90%–98% ee) by enantioconvergent hydrolysis using the reprogrammed variants. These findings provide a successful strategy for enhancing the enantioconvergence of native epoxide hydrolases through key single‐site mutation and more powerful enzyme tools for the enantioconvergent hydrolysis of styrene oxide scaffolds into single (R)‐enantiomers of chiral vicinal diols.
中文翻译:

重编程环氧水解酶,以提高苯乙烯氧化物支架水解中的对映体收敛性
通过环氧水解酶的对映体收敛水解是一种重要的邻位二醇合成的有前途的方法。但是,天然酶的区域选择性差会导致苯乙烯氧化物的酶促水解中对映体收敛性低。在此,根据结构信息重新设计了263号残基,并使用“优化的氨基酸字母”通过定点修饰构建了一个智能变体文库,以提高来自Vigna radiata(Vr EH2)的环氧水解酶的区域选择性。M263Q变体对间位异构体R异构体的区域选择性系数(r)与野生型相比,预取代的苯乙烯氧化物提高了40-63倍,变体M263V对对位取代的苯乙烯氧化物的R异构体也表现出更高的区域选择性,从而提高了苯乙烯氧化物支架水解中的对映体收敛性。结构上的洞察力表明263号残基在通过改变结合环境来调节底物结合构象中的关键作用。此外,亲核残基Asp101和环氧化物的两个碳原子之间的攻击距离差异增加,为区域选择性的提高提供了证据。几种易于合成的高价值邻位二醇(> 88%收率,90%–98% ee),使用重新编程的变体进行对映体收敛水解。这些发现为通过关键的单点突变增强天然环氧化物水解酶的对映体融合提供了成功的策略,并且提供了更强大的酶工具,用于将苯乙烯氧化物支架对映体水解为手性邻位二醇的单个(R)对映体。
更新日期:2020-11-04
中文翻译:

重编程环氧水解酶,以提高苯乙烯氧化物支架水解中的对映体收敛性
通过环氧水解酶的对映体收敛水解是一种重要的邻位二醇合成的有前途的方法。但是,天然酶的区域选择性差会导致苯乙烯氧化物的酶促水解中对映体收敛性低。在此,根据结构信息重新设计了263号残基,并使用“优化的氨基酸字母”通过定点修饰构建了一个智能变体文库,以提高来自Vigna radiata(Vr EH2)的环氧水解酶的区域选择性。M263Q变体对间位异构体R异构体的区域选择性系数(r)与野生型相比,预取代的苯乙烯氧化物提高了40-63倍,变体M263V对对位取代的苯乙烯氧化物的R异构体也表现出更高的区域选择性,从而提高了苯乙烯氧化物支架水解中的对映体收敛性。结构上的洞察力表明263号残基在通过改变结合环境来调节底物结合构象中的关键作用。此外,亲核残基Asp101和环氧化物的两个碳原子之间的攻击距离差异增加,为区域选择性的提高提供了证据。几种易于合成的高价值邻位二醇(> 88%收率,90%–98% ee),使用重新编程的变体进行对映体收敛水解。这些发现为通过关键的单点突变增强天然环氧化物水解酶的对映体融合提供了成功的策略,并且提供了更强大的酶工具,用于将苯乙烯氧化物支架对映体水解为手性邻位二醇的单个(R)对映体。











































 京公网安备 11010802027423号
京公网安备 11010802027423号