当前位置:
X-MOL 学术
›
Int. J. Quantum Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oriented external electric fields regulating the oxidation reaction of CH4 catalyzed by Mn‐corrolazine
International Journal of Quantum Chemistry ( IF 2.3 ) Pub Date : 2020-08-26 , DOI: 10.1002/qua.26443 Xiangqian Wang 1 , Haiyan Wang 1 , Lifeng Zheng 1 , Chun Zhu 1 , Jin‐Xia Liang 2
International Journal of Quantum Chemistry ( IF 2.3 ) Pub Date : 2020-08-26 , DOI: 10.1002/qua.26443 Xiangqian Wang 1 , Haiyan Wang 1 , Lifeng Zheng 1 , Chun Zhu 1 , Jin‐Xia Liang 2
Affiliation
|
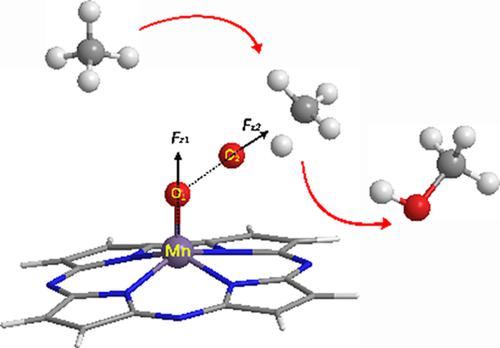
Mn‐corrolazine‐catalyzed CH4 oxidation, as well as the regulation of its oriented external electric fields (OEEFs), is systematically studied using the first‐principle calculations. Extensive density functional calculations show that the activation energy of CH4 oxidation catalyzed by Mn‐corrolazine is up to 38.5 kcal/mol in the field‐free condition. However, when the OEEF is applied, the reactant and the transition state of the oxidation reaction are stabilized, originating from an attractive interaction between the increased dipole moment and the applied fields. Furthermore, when the field is negative, the activation energy decreases as the field increases. Especially for the negative field along the intrinsic MnO reaction axis perpendicular to the corrolazine ring, of which the orientation is easily aligned in practical applications, when its intensity reaches −0.015 a.u., the activation energy of CH4 oxidation is reduced to 25.0 kcal/mol.
中文翻译:

定向外电场调节Mn-corrolazine催化CH4的氧化反应
使用第一性原理系统地研究了Mn-corrolazine催化的CH 4氧化及其定向外部电场(OEEF)的调节。大量的密度泛函计算表明,在无磁场条件下,Mn-corrolazine催化的CH 4氧化活化能高达38.5 kcal / mol。然而,当施加OEEF时,由于增加的偶极矩与所施加的场之间的吸引相互作用而使反应物和氧化反应的过渡态稳定。此外,当场为负时,激活能随着场的增加而降低。特别是对于沿着本征Mn的负场垂直于科拉嗪环的O反应轴,其取向在实际应用中易于对齐,当其强度达到-0.015 au时,CH 4氧化的活化能降低至25.0 kcal / mol。
更新日期:2020-08-26
中文翻译:

定向外电场调节Mn-corrolazine催化CH4的氧化反应
使用第一性原理系统地研究了Mn-corrolazine催化的CH 4氧化及其定向外部电场(OEEF)的调节。大量的密度泛函计算表明,在无磁场条件下,Mn-corrolazine催化的CH 4氧化活化能高达38.5 kcal / mol。然而,当施加OEEF时,由于增加的偶极矩与所施加的场之间的吸引相互作用而使反应物和氧化反应的过渡态稳定。此外,当场为负时,激活能随着场的增加而降低。特别是对于沿着本征Mn的负场垂直于科拉嗪环的O反应轴,其取向在实际应用中易于对齐,当其强度达到-0.015 au时,CH 4氧化的活化能降低至25.0 kcal / mol。











































 京公网安备 11010802027423号
京公网安备 11010802027423号