Cellular and Molecular Gastroenterology and Hepatology ( IF 7.1 ) Pub Date : 2020-08-27 , DOI: 10.1016/j.jcmgh.2020.08.009 Matthew Dent 1 , Krystal Hamorsky 2 , Thibaut Vausselin 3 , Jean Dubuisson 3 , Yoshinari Miyata 4 , Yoshio Morikawa 4 , Nobuyuki Matoba 5
|
Background & Aims
Infection with hepatitis C virus (HCV) remains a major cause of morbidity and mortality worldwide despite the recent advent of highly effective direct-acting antivirals. The envelope glycoproteins of HCV are heavily glycosylated with a high proportion of high-mannose glycans (HMGs), which serve as a shield against neutralizing antibodies and assist in the interaction with cell-entry receptors. However, there is no approved therapeutic targeting this potentially druggable biomarker.
Methods
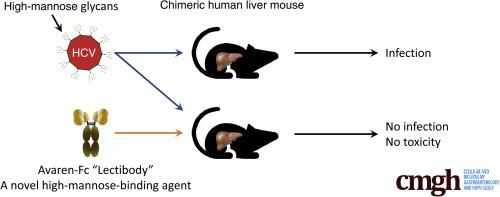
The anti-HCV activity of a fusion protein consisting of Avaren lectin and the fragment crystallizable (Fc) region of a human immunoglobulin G1 antibody, Avaren-Fc (AvFc) was evaluated through the use of in vitro neutralization assays as well as an in vivo challenge in a chimeric human liver (PXB) mouse model. Drug toxicity was assessed by histopathology, serum alanine aminotransferase, and mouse body weights.
Results
AvFc was capable of neutralizing cell culture–derived HCV in a genotype-independent manner, with 50% inhibitory concentration values in the low nanomolar range. Systemic administration of AvFc in a histidine-based buffer was well tolerated; after 11 doses every other day at 25 mg/kg there were no significant changes in body or liver weights or in blood human albumin or serum alanine aminotransferase activity. Gross necropsy and liver pathology confirmed the lack of toxicity. This regimen successfully prevented genotype 1a HCV infection in all animals, although an AvFc mutant lacking HMG binding activity failed.
Conclusions
These results suggest that targeting envelope HMGs is a promising therapeutic approach against HCV infection, and AvFc may provide a safe and efficacious means to prevent recurrent infection upon liver transplantation in HCV-related end-stage liver disease patients.
中文翻译:

Avaren-Fc Lectibody 在人肝嵌合小鼠模型中靶向 HCV 高甘露糖聚糖的安全性和有效性。
背景与目标
尽管最近出现了高效的直接作用抗病毒药物,但丙型肝炎病毒(HCV)感染仍然是全世界发病和死亡的主要原因。 HCV 的包膜糖蛋白被高比例的高甘露糖聚糖 (HMG) 严重糖基化,可作为抵御中和抗体的屏障,并有助于与细胞进入受体的相互作用。然而,目前还没有针对这种潜在可药物生物标志物的批准治疗方法。
方法
由 Avaren 凝集素和人免疫球蛋白 G1 抗体 Avaren-Fc (AvFc) 片段可结晶 (Fc) 区域组成的融合蛋白的抗 HCV 活性通过使用体外中和测定以及体内试验进行评估。嵌合人肝(PXB)小鼠模型中的挑战。通过组织病理学、血清丙氨酸转氨酶和小鼠体重评估药物毒性。
结果
AvFc 能够以与基因型无关的方式中和细胞培养物衍生的 HCV,在低纳摩尔范围内具有 50% 的抑制浓度值。在基于组氨酸的缓冲液中全身施用 AvFc 具有良好的耐受性;每隔一天服用 11 剂 25 mg/kg 后,体重或肝脏重量、血液人白蛋白或血清丙氨酸氨基转移酶活性没有显着变化。大体尸检和肝脏病理学证实没有毒性。尽管缺乏 HMG 结合活性的 AvFc 突变体失败了,但该方案成功预防了所有动物的基因型 1a HCV 感染。
结论
这些结果表明,靶向包膜HMG是一种有前途的抗HCV感染的治疗方法,而AvFc可能为预防HCV相关终末期肝病患者肝移植后的复发感染提供安全有效的手段。









































 京公网安备 11010802027423号
京公网安备 11010802027423号