当前位置:
X-MOL 学术
›
Tetrahedron Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Direct and practical Gilman-Speeter synthesis of 3,4-trisubstituted β-lactams via the Thorpe-Ingold effect
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2020-08-27 , DOI: 10.1016/j.tetlet.2020.152375 Maria Panagiotou , Vasileios Demos , Plato Α. Magriotis
中文翻译:

通过Thorpe-Ingold效应直接实用的Gilman-Speeter合成3,4-三取代的β-内酰胺
更新日期:2020-09-18
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2020-08-27 , DOI: 10.1016/j.tetlet.2020.152375 Maria Panagiotou , Vasileios Demos , Plato Α. Magriotis
|
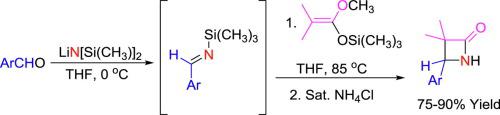
A highly efficient Gilman-Speeter synthesis of 3,4-trisubstituted β-lactams possessing a 4-aryl substituent is described, employing a direct, uncatalyzed Mannich reaction between TMS imines and TMS ketene acetals. The process avoids cryogenic conditions, making it more amenable to process-scale use than related methods for β-lactam synthesis. A Gilman-Speeter diastereoselective version using a sulfinyl imine and leading to homochiral sulfinyl β-aminoester is also presented.
中文翻译:

通过Thorpe-Ingold效应直接实用的Gilman-Speeter合成3,4-三取代的β-内酰胺
描述了具有TMS亚胺和TMS烯酮缩醛之间直接,未催化的曼尼希反应的高效的Gilman-Speeter合成具有4-芳基取代基的3,4-三取代的β-内酰胺的方法。该方法避免了低温条件,使其比β-内酰胺合成的相关方法更适合于工艺规模使用。还提出了使用亚磺酰基亚胺并导致同手性亚磺酰基β-氨基酯的Gilman-Speeter非对映选择性方案。










































 京公网安备 11010802027423号
京公网安备 11010802027423号