Structure ( IF 4.4 ) Pub Date : 2020-08-27 , DOI: 10.1016/j.str.2020.08.004 David Nicholson 1 , Thomas A Edwards 1 , Alex J O'Neill 1 , Neil A Ranson 1
|
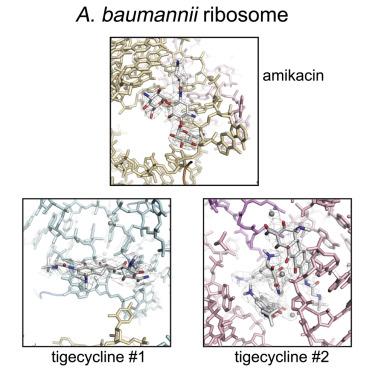
Acinetobacter baumannii is a Gram-negative bacterium primarily associated with hospital-acquired, often multidrug-resistant (MDR) infections. The ribosome-targeting antibiotics amikacin and tigecycline are among the limited arsenal of drugs available for treatment of such infections. We present high-resolution structures of the 70S ribosome from A. baumannii in complex with these antibiotics, as determined by cryoelectron microscopy. Comparison with the ribosomes of other bacteria reveals several unique structural features at functionally important sites, including around the exit of the polypeptide tunnel and the periphery of the subunit interface. The structures also reveal the mode and site of interaction of these drugs with the ribosome. This work paves the way for the design of new inhibitors of translation to address infections caused by MDR A. baumannii.
中文翻译:

来自人类病原体鲍曼不动杆菌的 70S 核糖体与临床相关抗生素复合物的结构。
鲍曼不动杆菌是一种革兰氏阴性菌,主要与医院获得性、通常是耐多药 (MDR) 感染有关。核糖体靶向抗生素阿米卡星和替加环素是可用于治疗此类感染的有限药物库。我们展示了来自A的 70S 核糖体的高分辨率结构。鲍曼不动杆菌与这些抗生素复合,由冷冻电子显微镜确定。与其他细菌的核糖体的比较揭示了在功能重要位点的几个独特的结构特征,包括多肽隧道出口周围和亚基界面的外围。这些结构还揭示了这些药物与核糖体相互作用的模式和部位。这项工作为设计新的翻译抑制剂以解决 MDR A引起的感染铺平了道路。鲍曼不动杆菌。











































 京公网安备 11010802027423号
京公网安备 11010802027423号