当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oxidative damage of proline residues by nitrate radicals (NO3˙): a kinetic and product study.
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020-08-26 , DOI: 10.1039/d0ob01337d Joses G Nathanael 1 , Jonathan M White , Annika Richter , Madison R Nuske , Uta Wille
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020-08-26 , DOI: 10.1039/d0ob01337d Joses G Nathanael 1 , Jonathan M White , Annika Richter , Madison R Nuske , Uta Wille
Affiliation
|
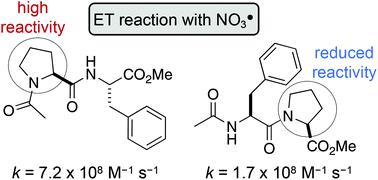
Tertiary amides, such as in N-acylated proline or N-methyl glycine residues, react rapidly with nitrate radicals (NO3˙) with absolute rate coefficients in the range of 4–7 × 108 M−1 s−1 in acetonitrile. The major pathway proceeds through oxidative electron transfer (ET) at nitrogen, whereas hydrogen abstraction is only a minor contributor under these conditions. However, steric hindrance at the amide, for example by alkyl side chains at the α-carbon, lowers the rate coefficient by up to 75%, indicating that NO3˙-induced oxidation of amide bonds proceeds through initial formation of a charge transfer complex. Furthermore, the rate of oxidative damage of proline and N-methyl glycine is significantly influenced by its position in a peptide. Thus, neighbouring peptide bonds, particularly in the N-direction, reduce the electron density at the tertiary amide, which slows down the rate of ET by up to one order of magnitude. The results from these model studies suggest that the susceptibility of proline residues in peptides to radical-induced oxidative damage should be considerably reduced, compared with the single amino acid.
中文翻译:

硝酸根(NO3˙)对脯氨酸残基的氧化损伤:动力学和产物研究。
叔酰胺,例如N-酰化脯氨酸或N-甲基甘氨酸残基,与硝酸根 (NO 3 ˙) 快速反应,在乙腈中的绝对速率系数范围为 4-7 × 10 8 M -1 s -1 。主要途径通过氮处的氧化电子转移 (ET) 进行,而在这些条件下,夺氢只是次要因素。然而,酰胺的空间位阻,例如α-碳上的烷基侧链,使速率系数降低高达 75%,表明 NO 3˙ 诱导的酰胺键氧化通过电荷转移复合物的初始形成进行。此外,脯氨酸和N-甲基甘氨酸的氧化损伤率显着受其在肽中的位置的影响。因此,相邻的肽键,特别是在N方向上,会降低叔酰胺的电子密度,从而将 ET 的速率降低多达一个数量级。这些模型研究的结果表明,与单一氨基酸相比,肽中脯氨酸残基对自由基诱导的氧化损伤的敏感性应大大降低。
更新日期:2020-09-16
中文翻译:

硝酸根(NO3˙)对脯氨酸残基的氧化损伤:动力学和产物研究。
叔酰胺,例如N-酰化脯氨酸或N-甲基甘氨酸残基,与硝酸根 (NO 3 ˙) 快速反应,在乙腈中的绝对速率系数范围为 4-7 × 10 8 M -1 s -1 。主要途径通过氮处的氧化电子转移 (ET) 进行,而在这些条件下,夺氢只是次要因素。然而,酰胺的空间位阻,例如α-碳上的烷基侧链,使速率系数降低高达 75%,表明 NO 3˙ 诱导的酰胺键氧化通过电荷转移复合物的初始形成进行。此外,脯氨酸和N-甲基甘氨酸的氧化损伤率显着受其在肽中的位置的影响。因此,相邻的肽键,特别是在N方向上,会降低叔酰胺的电子密度,从而将 ET 的速率降低多达一个数量级。这些模型研究的结果表明,与单一氨基酸相比,肽中脯氨酸残基对自由基诱导的氧化损伤的敏感性应大大降低。











































 京公网安备 11010802027423号
京公网安备 11010802027423号