Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A 96-well format microvascularized human lung-on-a-chip platform for microphysiological modeling of fibrotic diseases
Lab on a Chip ( IF 6.1 ) Pub Date : 2020-08-26 , DOI: 10.1039/d0lc00644k Joscelyn C Mejías 1 , Michael R Nelson 1 , Olivia Liseth 1 , Krishnendu Roy 1
Lab on a Chip ( IF 6.1 ) Pub Date : 2020-08-26 , DOI: 10.1039/d0lc00644k Joscelyn C Mejías 1 , Michael R Nelson 1 , Olivia Liseth 1 , Krishnendu Roy 1
Affiliation
|
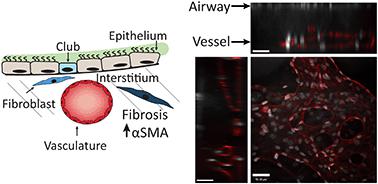
Development of organoids and microfluidic on-chip models has enabled studies of organ-level disease pathophysiologies in vitro. However, current lung-on-a-chip platforms are primarily monolayer epithelial–endothelial co-cultures, separated by a thin membrane, lacking microvasculature-networks or interstitial-fibroblasts. Here we report the design, microfabrication, and characterization of a unique microphysiological on-chip device that recapitulates the human lung interstitium–airway interface through a 3D vascular network, and normal or diseased fibroblasts encapsulated within a fibrin-collagen hydrogel underneath an airlifted airway epithelium. By incorporating fibroblasts from donors with idiopathic pulmonary fibrosis (IPF), or healthy-donor fibroblasts treated with TGF-β1, we successfully created a fibrotic, alpha smooth muscle actin (αSMA)-positive disease phenotype which led to fibrosis-like transformation in club cells and ciliated cells in the airway. Using this device platform, we further modeled the cystic fibrosis (CF) epithelium and recruitment of neutrophils to the vascular networks. Our results suggest that this microphysiological model of the human lung could enable more pathophysiologically relevant studies of complex pulmonary diseases.
中文翻译:

96孔格式的微血管化人肺芯片平台,用于纤维化疾病的微生理建模
类器官和微流控芯片模型的开发使得能够进行体外器官水平疾病病理生理学的研究。但是,当前的“单芯片肺”平台主要是单层上皮-内皮共培养,被薄膜隔开,缺乏微脉管网络或间质成纤维细胞。在这里,我们报告了一种独特的微生理芯片上设备的设计,微制造和表征,该设备通过3D血管网络概括了人类肺间质-气道界面,以及正常或患病的成纤维细胞封装在气举上皮下的纤维蛋白-胶原水凝胶中。通过合并患有特发性肺纤维化(IPF)的供体的成纤维细胞或接受TGF-β1治疗的健康供体的成纤维细胞,我们成功创建了纤维化的α平滑肌肌动蛋白(αSMA)阳性疾病表型,导致了俱乐部中的纤维化样转化气道中的细胞和纤毛细胞。使用该设备平台,我们进一步对囊性纤维化(CF)上皮和嗜中性粒细胞募集到血管网络进行了建模。我们的结果表明,这种人类肺部的微生理模型可以使更多复杂的肺部疾病的病理生理学相关研究成为可能。
更新日期:2020-09-29
中文翻译:

96孔格式的微血管化人肺芯片平台,用于纤维化疾病的微生理建模
类器官和微流控芯片模型的开发使得能够进行体外器官水平疾病病理生理学的研究。但是,当前的“单芯片肺”平台主要是单层上皮-内皮共培养,被薄膜隔开,缺乏微脉管网络或间质成纤维细胞。在这里,我们报告了一种独特的微生理芯片上设备的设计,微制造和表征,该设备通过3D血管网络概括了人类肺间质-气道界面,以及正常或患病的成纤维细胞封装在气举上皮下的纤维蛋白-胶原水凝胶中。通过合并患有特发性肺纤维化(IPF)的供体的成纤维细胞或接受TGF-β1治疗的健康供体的成纤维细胞,我们成功创建了纤维化的α平滑肌肌动蛋白(αSMA)阳性疾病表型,导致了俱乐部中的纤维化样转化气道中的细胞和纤毛细胞。使用该设备平台,我们进一步对囊性纤维化(CF)上皮和嗜中性粒细胞募集到血管网络进行了建模。我们的结果表明,这种人类肺部的微生理模型可以使更多复杂的肺部疾病的病理生理学相关研究成为可能。









































 京公网安备 11010802027423号
京公网安备 11010802027423号