当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Syntheses of 2-Iminoindolin-3-ones and 2-Alknyl-2,3-dihydroquinazolin-4(1H)-ones from 3-Diazoindolin-2-imines.
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-08-26 , DOI: 10.1021/acs.joc.0c01548 Zhenwei Lin 1 , Jing Qian 1 , Ping Lu 1 , Yanguang Wang 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-08-26 , DOI: 10.1021/acs.joc.0c01548 Zhenwei Lin 1 , Jing Qian 1 , Ping Lu 1 , Yanguang Wang 1
Affiliation
|
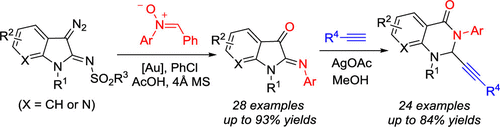
3-Diazoindolin-2-imines reacted with nitrones to furnish 2-iminoindolin-3-ones through a Au(I)-catalyzed cascade oxygen transfer/imine exchange process. The prepared 2-iminoindolin-3-ones could be further transformed into 2-alknyl-2,3-dihydroquinazolin-4(1H)-ones through a Ag(I)-catalyzed reaction with terminal alkynes. A MeOH-triggered ring expansion mechanism involving cyclic iminium formation and nucleophilic addition is proposed for this novel alkynylation reaction. This two-step procedure provides a general and convenient approach to 2-alknyl-2,3-dihydroquinazolin-4(1H)-ones, which are privileged structures in medicinal chemistry.
中文翻译:

由3-Diazoindolin-2-imines合成2-Iminoindolin-3-ones和2-Alknyl-2,3-dihydroquinazolin-4(1H)-ones from 3-Diazoindolin-2-imines。
3-重氮吲哚-2-亚胺与硝酮反应,通过Au(I)催化的级联氧转移/亚胺交换过程提供了2-iminoindolin-3-ones。制备的2-亚氨基吲哚-3-酮可通过Ag(I)催化的与末端炔烃的反应进一步转化为2-炔基-2,3-二氢喹唑啉-4(1 H)-酮。针对这种新型的炔基化反应,提出了一种由甲醇引发的环扩展机制,该机制涉及环状亚胺基的形成和亲核加成。此两步过程为2-炔基-2,3-二氢喹唑啉-4(1 H)-酮提供了一种通用且便捷的方法,这是药物化学中的优先结构。
更新日期:2020-09-20
中文翻译:

由3-Diazoindolin-2-imines合成2-Iminoindolin-3-ones和2-Alknyl-2,3-dihydroquinazolin-4(1H)-ones from 3-Diazoindolin-2-imines。
3-重氮吲哚-2-亚胺与硝酮反应,通过Au(I)催化的级联氧转移/亚胺交换过程提供了2-iminoindolin-3-ones。制备的2-亚氨基吲哚-3-酮可通过Ag(I)催化的与末端炔烃的反应进一步转化为2-炔基-2,3-二氢喹唑啉-4(1 H)-酮。针对这种新型的炔基化反应,提出了一种由甲醇引发的环扩展机制,该机制涉及环状亚胺基的形成和亲核加成。此两步过程为2-炔基-2,3-二氢喹唑啉-4(1 H)-酮提供了一种通用且便捷的方法,这是药物化学中的优先结构。











































 京公网安备 11010802027423号
京公网安备 11010802027423号