当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Unusual Acetonitrile Adduct Formed via Photolysis of 4'-Chloro-2-Hydroxybiphenyl in Aqueous Solution.
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-08-25 , DOI: 10.1021/acs.joc.0c01115 Xiting Zhang 1, 2 , Yan Guo 1 , Erin Dallin 3 , Jiani Ma 1 , Mingdong Dai 1 , David Lee Phillips 2
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-08-25 , DOI: 10.1021/acs.joc.0c01115 Xiting Zhang 1, 2 , Yan Guo 1 , Erin Dallin 3 , Jiani Ma 1 , Mingdong Dai 1 , David Lee Phillips 2
Affiliation
|
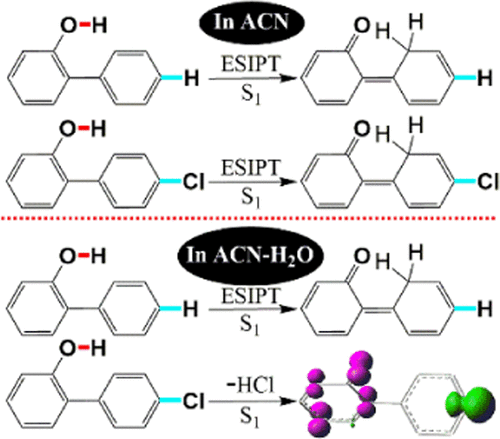
In this work, 2,4′-dichlorobiphenyl (1) yielded 4′-chloro-2-hydroxybiphenyl (2) after photolysis in neutral acetonitrile aqueous (ACN–H2O) solutions. Ultrafast spectroscopic measurements and density functional theory (DFT) computations were performed for 2 in ACN and ACN–H2O (v/v, 1:1). These results were compared with previously published results for 2-hydroxybiphenyl (3). The counterparts 2 and 3 went through a singlet excited state intramolecular proton transfer (ESIPT) in ACN but behaved differently in ACN–H2O with a dehydrochlorination process occurring for 2 and an ESIPT taking place for 3. Computational results indicate that the phenol O–H bond elongates after photoexcitation to induce a concerted asynchronous process with the C–Cl bond increasing first followed by HCl elimination. A biradical intermediate (IM1) is then formed with some spin located at the phenyl 4′-C radical that appears to favor a hydrogen atom transfer (HAT) process and some spin located on phenoxyl that appears to prefer a subsequent •CH2CN radical rebound. The hydrogen bond promotes HCl elimination, while this is disfavored for ESIPT, making 4′-Cl extrusion the predominant process in ACN–H2O solutions. The mechanistic investigations have fundamental and significant implications for the understanding of polychlorinated biphenyl photolysis in an aqueous environment and hence the photodegradation of these kinds of pollutants in the natural environment.
中文翻译:

通过在水溶液中光解4'-氯-2-羟基联苯形成异常的乙腈加合物。
在这项工作中,在中性乙腈水溶液(ACN–H 2 O)中光解后,2,4'-二氯联苯(1)产生4'-氯-2-羟基联苯(2)。在ACN和ACN-H 2 O(v / v,1:1)中进行了2种超快光谱测量和密度泛函理论(DFT)计算。将这些结果与先前公布的2-羟基联苯(3)结果进行了比较。同行2和3经历了在ACN单重激发态分子内质子转移(ESIPT),但在ACN-H表现不同2 ö与脱氯化氢工艺中出现对2和ESIPT进行3。计算结果表明,光激发后,苯酚的O–H键伸长,引发协同的异步过程,首先增加C–Cl键,然后消除HCl。然后形成双自由基中间体(IM1),其中一些自旋位于苯基4'-C自由基上,这似乎有利于氢原子转移(HAT)过程,而某些自旋位于苯氧基上,似乎更喜欢随后的•CH 2 CN基团反弹。氢键促进HCl的消除,而这对ESIPT不利,使4'-Cl挤出成为ACN–H 2中的主要过程O解决方案。机理研究对于理解多氯联苯在水环境中的光解以及因此在自然环境中对这类污染物的光降解具有根本性和重要的意义。
更新日期:2020-09-20
中文翻译:

通过在水溶液中光解4'-氯-2-羟基联苯形成异常的乙腈加合物。
在这项工作中,在中性乙腈水溶液(ACN–H 2 O)中光解后,2,4'-二氯联苯(1)产生4'-氯-2-羟基联苯(2)。在ACN和ACN-H 2 O(v / v,1:1)中进行了2种超快光谱测量和密度泛函理论(DFT)计算。将这些结果与先前公布的2-羟基联苯(3)结果进行了比较。同行2和3经历了在ACN单重激发态分子内质子转移(ESIPT),但在ACN-H表现不同2 ö与脱氯化氢工艺中出现对2和ESIPT进行3。计算结果表明,光激发后,苯酚的O–H键伸长,引发协同的异步过程,首先增加C–Cl键,然后消除HCl。然后形成双自由基中间体(IM1),其中一些自旋位于苯基4'-C自由基上,这似乎有利于氢原子转移(HAT)过程,而某些自旋位于苯氧基上,似乎更喜欢随后的•CH 2 CN基团反弹。氢键促进HCl的消除,而这对ESIPT不利,使4'-Cl挤出成为ACN–H 2中的主要过程O解决方案。机理研究对于理解多氯联苯在水环境中的光解以及因此在自然环境中对这类污染物的光降解具有根本性和重要的意义。











































 京公网安备 11010802027423号
京公网安备 11010802027423号