当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Can Selenoenzymes Resist Electrophilic Modification? Evidence from Thioredoxin Reductase and a Mutant Containing α-Methylselenocysteine.
Biochemistry ( IF 2.9 ) Pub Date : 2020-08-26 , DOI: 10.1021/acs.biochem.0c00608 Emma J Ste Marie 1, 2 , Robert J Wehrle 3 , Daniel J Haupt 2 , Neil B Wood 4 , Albert van der Vliet 5 , Michael J Previs 4 , Douglas S Masterson 3 , Robert J Hondal 1, 2
Biochemistry ( IF 2.9 ) Pub Date : 2020-08-26 , DOI: 10.1021/acs.biochem.0c00608 Emma J Ste Marie 1, 2 , Robert J Wehrle 3 , Daniel J Haupt 2 , Neil B Wood 4 , Albert van der Vliet 5 , Michael J Previs 4 , Douglas S Masterson 3 , Robert J Hondal 1, 2
Affiliation
|
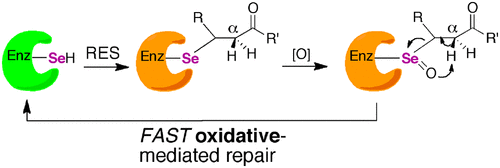
Selenocysteine (Sec) is the 21st proteogenic amino acid in the genetic code. Incorporation of Sec into proteins is a complex and bioenergetically costly process that evokes the following question: “Why did nature choose selenium?” An answer that has emerged over the past decade is that Sec confers resistance to irreversible oxidative inactivation by reactive oxygen species. Here, we explore the question of whether this concept can be broadened to include resistance to reactive electrophilic species (RES) because oxygen and related compounds are merely a subset of RES. To test this hypothesis, we inactivated mammalian thioredoxin reductase (Sec-TrxR), a mutant containing α-methylselenocysteine [(αMe)Sec-TrxR], and a cysteine ortholog TrxR (Cys-TrxR) with various electrophiles, including acrolein, 4-hydroxynonenal, and curcumin. Our results show that the acrolein-inactivated Sec-TrxR and the (αMe)Sec-TrxR mutant could regain 25% and 30% activity, respectively, when incubated with 2 mM H2O2 and 5 mM imidazole. In contrast, Cys-TrxR did not regain activity under the same conditions. We posit that Sec enzymes can undergo a repair process via β-syn selenoxide elimination that ejects the electrophile, leaving the enzyme in the oxidized selenosulfide state. (αMe)Sec-TrxR was created by incorporating the non-natural amino acid (αMe)Sec into TrxR by semisynthesis and allowed for rigorous testing of our hypothesis. This Sec derivative enables higher resistance to both oxidative and electrophilic inactivation because it lacks a backbone Cα-H, which prevents loss of selenium through the formation of dehydroalanine. This is the first time this unique amino acid has been incorporated into an enzyme and is an example of state-of-the-art protein engineering.
中文翻译:

硒酶可以抵抗亲电修饰吗?来自硫氧还蛋白还原酶和含有α-甲基硒代半胱氨酸的突变体的证据。
硒代半胱氨酸(Sec)是遗传密码中的第21个蛋白氨基酸。将Sec掺入蛋白质是一个复杂且耗费能源的生物过程,引起了以下问题:“自然界为什么选择硒?” 在过去十年中出现的一个答案是,Sec赋予了对活性氧不可逆的氧化失活的抵抗力。在这里,我们探讨了这个概念是否可以扩展为包括对反应性亲电物种(RES)的抵抗力的问题,因为氧气和相关化合物仅仅是RES的子集。为了验证这一假设,我们用各种亲电试剂(包括丙烯醛,4-羟基壬醛和姜黄素。2 O 2和5 mM咪唑。相反,Cys-TrxR在相同条件下未恢复活性。我们认为,Sec酶可以通过β-顺硒酸消除消除修复过程,从而喷射亲电试剂,从而使酶处于氧化硒代硫化物状态。(αMe)Sec-TrxR是通过半合成将非天然氨基酸(αMe)Sec掺入TrxR中而创建的,可用于严格检验我们的假设。此二段衍生物能够既氧化和电灭活更高的电阻,因为它缺少一个骨干Ç α-H,它通过形成脱氢丙氨酸防止硒损失。这是这种独特的氨基酸首次被掺入酶中,并且是最新蛋白质工程的一个例子。
更新日期:2020-09-15
中文翻译:

硒酶可以抵抗亲电修饰吗?来自硫氧还蛋白还原酶和含有α-甲基硒代半胱氨酸的突变体的证据。
硒代半胱氨酸(Sec)是遗传密码中的第21个蛋白氨基酸。将Sec掺入蛋白质是一个复杂且耗费能源的生物过程,引起了以下问题:“自然界为什么选择硒?” 在过去十年中出现的一个答案是,Sec赋予了对活性氧不可逆的氧化失活的抵抗力。在这里,我们探讨了这个概念是否可以扩展为包括对反应性亲电物种(RES)的抵抗力的问题,因为氧气和相关化合物仅仅是RES的子集。为了验证这一假设,我们用各种亲电试剂(包括丙烯醛,4-羟基壬醛和姜黄素。2 O 2和5 mM咪唑。相反,Cys-TrxR在相同条件下未恢复活性。我们认为,Sec酶可以通过β-顺硒酸消除消除修复过程,从而喷射亲电试剂,从而使酶处于氧化硒代硫化物状态。(αMe)Sec-TrxR是通过半合成将非天然氨基酸(αMe)Sec掺入TrxR中而创建的,可用于严格检验我们的假设。此二段衍生物能够既氧化和电灭活更高的电阻,因为它缺少一个骨干Ç α-H,它通过形成脱氢丙氨酸防止硒损失。这是这种独特的氨基酸首次被掺入酶中,并且是最新蛋白质工程的一个例子。











































 京公网安备 11010802027423号
京公网安备 11010802027423号