当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Spectroscopic Identification of Peptide Chemistry in the Caulobacter crescentus Holdfast.
Biochemistry ( IF 2.9 ) Pub Date : 2020-08-26 , DOI: 10.1021/acs.biochem.0c00625 Alex Nyarko 1 , Saranshu Singla 1 , Hazel A Barton 2 , Ali Dhinojwala 1
Biochemistry ( IF 2.9 ) Pub Date : 2020-08-26 , DOI: 10.1021/acs.biochem.0c00625 Alex Nyarko 1 , Saranshu Singla 1 , Hazel A Barton 2 , Ali Dhinojwala 1
Affiliation
|
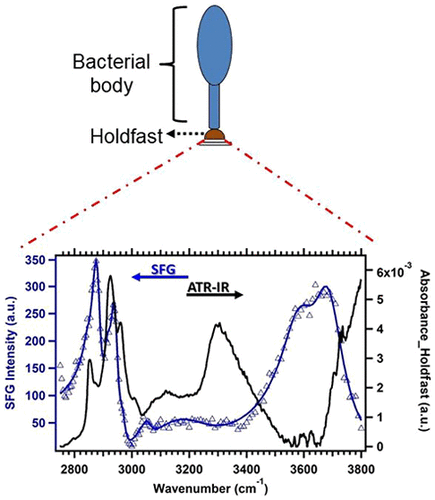
The bacterium Caulobacter crescentus is known to attach irreversibly to underwater surfaces by utilizing an adhesive structure called the holdfast, which exhibits the greatest known adhesive strength of any organism. The very small size of the holdfast (∼400 nm wide and ∼40 nm high) has made direct chemical analysis difficult, and its structure remains poorly understood. In this study, we employ spectroscopic techniques, including attenuated total reflection infrared spectroscopy (ATR-IR) and X-ray photoelectron spectroscopy, to probe holdfast chemistry. The data indicate the presence of a peptide signal within the holdfast polymer. By comparing the ATR-IR spectrum of the holdfast to peptidoglycan spectra from other bacterial species, we demonstrate the similarity of the holdfast chemistry to that of peptidoglycan, suggesting peptide cross-linking may play a role in holdfast architecture. To probe the molecular groups at the interface, surface-sensitive sum frequency generation spectroscopy was used to show that aromatic and hydroxyl groups related to this protein content at the adhesive interface could be playing a crucial role in adhesion. On the basis of these results, we propose a model of the holdfast architecture with similarities to the peptide cross-linking observed in the peptidoglycan polymer of the bacterial cell wall. These results not only provide information about the development of adhesives that could be based on holdfast chemical architecture but also reveal a potentially yet unexplored biosynthetic pathway in holdfast synthesis that has not yet been revealed by genetic approaches, thereby opening up a potentially new avenue of research in holdfast synthesis.
中文翻译:

在新月形杆菌Holdfast中肽化学的光谱鉴定。
细菌柄杆菌新月已知通过使用一种称为“坚牢牢牢”的粘合结构,可逆性地将其不可逆地附着在水下表面上,该结构表现出任何生物体已知的最大粘合强度。固定剂的尺寸非常小(宽约400 nm,高约40 nm),使得直接化学分析变得困难,并且其结构仍知之甚少。在这项研究中,我们采用光谱技术,包括衰减全反射红外光谱(ATR-IR)和X射线光电子能谱,来探测快速化学。数据表明在固定聚合物中存在肽信号。通过比较固定细菌的ATR-IR光谱与其他细菌种类的肽聚糖光谱,我们证明了固定化学与肽聚糖化学的相似性,提示肽的交联可能在保持结构中起作用。为了探测界面上的分子基团,使用了表面敏感的总和频率产生光谱法来显示与该蛋白质含量相关的芳族基团和羟基在胶粘剂界面上可能起着至关重要的作用。基于这些结果,我们提出了一种具有与细菌细胞壁的肽聚糖聚合物中观察到的肽交联相似的保持结构的模型。这些结果不仅提供了有关可以基于快速化学结构的胶粘剂开发的信息,而且还揭示了在快速合成中尚未被遗传方法揭示的潜在尚未开发的生物合成途径,
更新日期:2020-09-22
中文翻译:

在新月形杆菌Holdfast中肽化学的光谱鉴定。
细菌柄杆菌新月已知通过使用一种称为“坚牢牢牢”的粘合结构,可逆性地将其不可逆地附着在水下表面上,该结构表现出任何生物体已知的最大粘合强度。固定剂的尺寸非常小(宽约400 nm,高约40 nm),使得直接化学分析变得困难,并且其结构仍知之甚少。在这项研究中,我们采用光谱技术,包括衰减全反射红外光谱(ATR-IR)和X射线光电子能谱,来探测快速化学。数据表明在固定聚合物中存在肽信号。通过比较固定细菌的ATR-IR光谱与其他细菌种类的肽聚糖光谱,我们证明了固定化学与肽聚糖化学的相似性,提示肽的交联可能在保持结构中起作用。为了探测界面上的分子基团,使用了表面敏感的总和频率产生光谱法来显示与该蛋白质含量相关的芳族基团和羟基在胶粘剂界面上可能起着至关重要的作用。基于这些结果,我们提出了一种具有与细菌细胞壁的肽聚糖聚合物中观察到的肽交联相似的保持结构的模型。这些结果不仅提供了有关可以基于快速化学结构的胶粘剂开发的信息,而且还揭示了在快速合成中尚未被遗传方法揭示的潜在尚未开发的生物合成途径,











































 京公网安备 11010802027423号
京公网安备 11010802027423号