当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Sr2CoTaO6 Double Perovskite Oxide as a Novel Visible-Light-Absorbing Bifunctional Photocatalyst for Photocatalytic Oxygen and Hydrogen Evolution Reactions
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2020-08-25 , DOI: 10.1021/acssuschemeng.0c05237 Ahmed Mahmoud Idris 1, 2 , Taifeng Liu 3 , Jafar Hussain Shah 1, 2 , Hongxian Han 1, 2 , Can Li 1
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2020-08-25 , DOI: 10.1021/acssuschemeng.0c05237 Ahmed Mahmoud Idris 1, 2 , Taifeng Liu 3 , Jafar Hussain Shah 1, 2 , Hongxian Han 1, 2 , Can Li 1
Affiliation
|
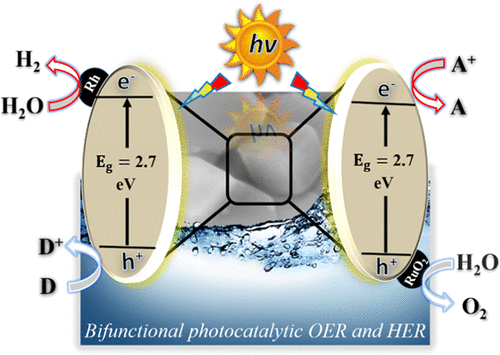
The development of efficient and stable semiconductor photocatalysts for water splitting is regarded as a viable means to produce renewable hydrogen using abundant solar energy. Although some oxide semiconductors have already been demonstrated as efficient and stable photocatalysts for overall water splitting under UV light irradiation, no oxide semiconductor photocatalysts have been reported so far to split water efficiently under visible light irradiation. Hence, screening of visible-light-absorbing oxide semiconductors is highly demanded for photocatalytic water splitting. Herein, Sr2CoTaO6 double perovskite oxide has been identified as a visible-light-absorbing semiconductor photocatalyst with an electronic band structure suitable for overall water splitting by density of states (DOS) analysis. To demonstrate the capability of photocatalytic water splitting properties of Sr2CoTaO6, cubic-shaped Sr2CoTaO6-F and irregular-shaped Sr2CoTaO6-S were synthesized by the facile flux method (F) and the conventional high-temperature solid-state reaction method (S). The experimental analysis of the band structure indicates that the synthesized Sr2CoTaO6 is an n-type semiconductor with a bandgap of 2.7 eV, and the minimum of conduction band (CB) positions and the maximum of valence band (VB) positions are located at −0.87 and +1.83 V vs normal hydrogen electrode (NHE, pH = 7), respectively. Sr2CoTaO6-F showed much higher photocatalytic oxygen evolution reaction (OER) and hydrogen evolution reaction (HER) activities than Sr2CoTaO6-S, indicating the advantage of the flux method in synthesizing double perovskite oxides in control of crystallinity and morphology. Without loading any cocatalysts, Sr2CoTaO6-F showed bifunctional photocatalytic oxygen evolution reaction (OER) and hydrogen evolution reaction (HER) activities in the presence of sacrificial reagents under visible light irradiation, which is rarely reported for metal-oxide-based photocatalysts. The photocatalytic OER and HER activities could be further enhanced by loading RuO2 and Rh cocatalysts, respectively. This work further supports that visible-light-absorbing double perovskite oxide semiconductors are promising candidates worth exploring for photocatalytic water splitting.
中文翻译:

Sr 2 CoTaO 6双钙钛矿氧化物作为新型可见光吸收双功能光催化剂,用于光催化氧和氢的生成反应
用于水分解的高效稳定的半导体光催化剂的开发被认为是利用大量太阳能生产可再生氢的可行方法。尽管已经证明某些氧化物半导体是在紫外光照射下用于整体水分解的有效和稳定的光催化剂,但是迄今为止,还没有报道过氧化物半导体光催化剂在可见光照射下能有效地将水分解。因此,对于光催化水分解,强烈要求屏蔽可见光吸收性氧化物半导体。在此,Sr 2 CoTaO 6双钙钛矿氧化物已被鉴定为具有电子带结构的可见光吸收半导体光催化剂,该电子带结构适于通过状态密度(DOS)分析进行整体水分解。为了证明Sr 2 CoTaO 6的光催化水分解性能,通过方便通量法(F)和常规高温合成了立方形状的Sr 2 CoTaO 6 -F和不规则形状的Sr 2 CoTaO 6 -S。固态反应方法(S)。能带结构的实验分析表明,合成的Sr 2 CoTaO 6是带隙为2.7 eV的n型半导体,相对于普通氢电极(NHE,pH),导带(CB)位置的最小值和价带(VB)位置的最大值位于-0.87和+1.83 V = 7)。与Sr 2 CoTaO 6 -S相比,Sr 2 CoTaO 6 -F表现出更高的光催化氧释放反应(OER)和氢释放反应(HER)活性,表明助熔剂法在控制钙钛矿氧化物的结晶度和形貌方面的优势。 。在不添加任何助催化剂的情况下,Sr 2 CoTaO 6-F在可见光照射下在牺牲试剂存在下显示双功能光催化氧释放反应(OER)和氢释放反应(HER)的活性,这对于基于金属氧化物的光催化剂鲜有报道。分别加入RuO 2和Rh助催化剂可以进一步提高光催化OER和HER活性。这项工作进一步支持了可见光吸收型钙钛矿氧化物半导体是有前途的候选物,值得光催化分解水。
更新日期:2020-09-21
中文翻译:

Sr 2 CoTaO 6双钙钛矿氧化物作为新型可见光吸收双功能光催化剂,用于光催化氧和氢的生成反应
用于水分解的高效稳定的半导体光催化剂的开发被认为是利用大量太阳能生产可再生氢的可行方法。尽管已经证明某些氧化物半导体是在紫外光照射下用于整体水分解的有效和稳定的光催化剂,但是迄今为止,还没有报道过氧化物半导体光催化剂在可见光照射下能有效地将水分解。因此,对于光催化水分解,强烈要求屏蔽可见光吸收性氧化物半导体。在此,Sr 2 CoTaO 6双钙钛矿氧化物已被鉴定为具有电子带结构的可见光吸收半导体光催化剂,该电子带结构适于通过状态密度(DOS)分析进行整体水分解。为了证明Sr 2 CoTaO 6的光催化水分解性能,通过方便通量法(F)和常规高温合成了立方形状的Sr 2 CoTaO 6 -F和不规则形状的Sr 2 CoTaO 6 -S。固态反应方法(S)。能带结构的实验分析表明,合成的Sr 2 CoTaO 6是带隙为2.7 eV的n型半导体,相对于普通氢电极(NHE,pH),导带(CB)位置的最小值和价带(VB)位置的最大值位于-0.87和+1.83 V = 7)。与Sr 2 CoTaO 6 -S相比,Sr 2 CoTaO 6 -F表现出更高的光催化氧释放反应(OER)和氢释放反应(HER)活性,表明助熔剂法在控制钙钛矿氧化物的结晶度和形貌方面的优势。 。在不添加任何助催化剂的情况下,Sr 2 CoTaO 6-F在可见光照射下在牺牲试剂存在下显示双功能光催化氧释放反应(OER)和氢释放反应(HER)的活性,这对于基于金属氧化物的光催化剂鲜有报道。分别加入RuO 2和Rh助催化剂可以进一步提高光催化OER和HER活性。这项工作进一步支持了可见光吸收型钙钛矿氧化物半导体是有前途的候选物,值得光催化分解水。











































 京公网安备 11010802027423号
京公网安备 11010802027423号