当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective Formal Arylation of (7‐Aza)isatylidene Malononitriles with α′‐Alkylidene‐2‐cyclohexenones
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-08-25 , DOI: 10.1002/adsc.202000900 Qing He 1 , Zhen‐Hong Yang 1 , Jing Yang 2 , Wei Du 1 , Ying‐Chun Chen 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-08-25 , DOI: 10.1002/adsc.202000900 Qing He 1 , Zhen‐Hong Yang 1 , Jing Yang 2 , Wei Du 1 , Ying‐Chun Chen 1
Affiliation
|
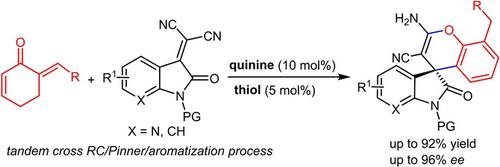
An asymmetric intermolecular Rauhut‐Currier reaction of α′‐alkylidene 2‐cyclohexenones and (7‐aza)isatinylidene malononitriles is realized under the double activation catalysis of natural quinine and 2‐mercaptobenzoic acid, finally affording formal 3‐arylated spiro (7‐aza)oxindole derivatives in fair to excellent enantioselectivity after a tandem cyclization/aromatization process (up to 96% ee).
中文翻译:

(7-氮杂)异亚丙基丙二腈与α'-亚烷基-2-环己烯酮的对映选择性形式化
在天然奎宁和2-巯基苯甲酸的双重活化催化下,实现了α'-亚烷基2-环己烯酮和(7-氮杂)异亚丙基丙二腈的不对称分子间Rauhut-Currier反应,最终得到了正式的3-芳基螺(7-氮杂)吲哚衍生物在串联环化/芳构化过程后(高达ee高达96%)具有良好的对映选择性。
更新日期:2020-10-26
中文翻译:

(7-氮杂)异亚丙基丙二腈与α'-亚烷基-2-环己烯酮的对映选择性形式化
在天然奎宁和2-巯基苯甲酸的双重活化催化下,实现了α'-亚烷基2-环己烯酮和(7-氮杂)异亚丙基丙二腈的不对称分子间Rauhut-Currier反应,最终得到了正式的3-芳基螺(7-氮杂)吲哚衍生物在串联环化/芳构化过程后(高达ee高达96%)具有良好的对映选择性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号