当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Polycyclic Furan and Chromene Derivatives via Cascade Reactions Enabled by Cleavage of Multiple C(sp3)−F Bonds
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-08-25 , DOI: 10.1002/adsc.202000660 Ting Xie 1 , Chen Zhang 1 , Si‐Xuan Zhang 1 , Weidong Rao 2 , Haiyan Xu 3 , Zhi‐Liang Shen 1 , Xue‐Qiang Chu 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-08-25 , DOI: 10.1002/adsc.202000660 Ting Xie 1 , Chen Zhang 1 , Si‐Xuan Zhang 1 , Weidong Rao 2 , Haiyan Xu 3 , Zhi‐Liang Shen 1 , Xue‐Qiang Chu 1
Affiliation
|
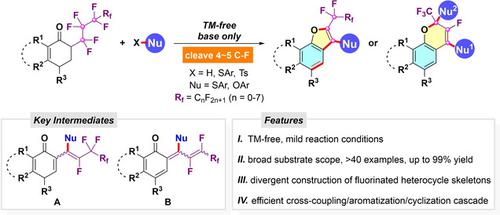
The strong and unreactive C−F bond makes polyfluorocarbons extremely long‐lived and potentially toxic. A successive selective and controllable C(sp3)−F functionalization of polyfluoroalkylated ketones with S‐ and O‐nucleophiles to enable efficient synthesis of pharmaceutically important fluorine‐ and sulfur‐containing polycyclic furan and chromene derivatives under transition metal‐free conditions is demonstrated here. The combination of C−S/C−O coupling, aromatization, and cyclization cascade contribute to the accurate four/five C(sp3)−F bond cleavage at two different sites of perfluoroalkyl chain. The formation of reactive quinoid intermediates and the necessity of using TBAB (tetrabutylammonium bromide) as additive and Cs2CO3 as base were identified by detailed mechanistic studies.
中文翻译:

通过裂解多个C(sp3)-F键实现的级联反应合成多环呋喃和二烯衍生物
牢固且无反应的C-F键使聚碳氟化合物寿命极长,并且可能具有毒性。本文展示了具有S和O亲核试剂的多氟烷基化酮的连续选择性和可控C(sp 3)-F功能化,可在无过渡金属的条件下有效合成重要的药学上重要的含氟和硫的多环呋喃和亚甲基苯衍生物。 。C-S / C-O偶联,芳构化和环化级联的组合有助于形成精确的4/5 C(sp 3)-F键在全氟烷基链的两个不同位点裂解。通过详细的机理研究,确定了反应性醌型中间体的形成以及使用TBAB(溴化四丁基铵)作为添加剂和Cs 2 CO 3作为碱的必要性。
更新日期:2020-08-25
中文翻译:

通过裂解多个C(sp3)-F键实现的级联反应合成多环呋喃和二烯衍生物
牢固且无反应的C-F键使聚碳氟化合物寿命极长,并且可能具有毒性。本文展示了具有S和O亲核试剂的多氟烷基化酮的连续选择性和可控C(sp 3)-F功能化,可在无过渡金属的条件下有效合成重要的药学上重要的含氟和硫的多环呋喃和亚甲基苯衍生物。 。C-S / C-O偶联,芳构化和环化级联的组合有助于形成精确的4/5 C(sp 3)-F键在全氟烷基链的两个不同位点裂解。通过详细的机理研究,确定了反应性醌型中间体的形成以及使用TBAB(溴化四丁基铵)作为添加剂和Cs 2 CO 3作为碱的必要性。









































 京公网安备 11010802027423号
京公网安备 11010802027423号