当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
α‐Amino Acids Mediated C−C Double Bonds Cleavage in Diastereoselective Synthesis of Aza‐Spirocyclic Pyrazolones
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-08-25 , DOI: 10.1002/adsc.202000884 Annapurna Awasthi 1, 2 , Pushpendra Yadav 1, 2 , Virendra Kumar 3 , Dharmendra Kumar Tiwari 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-08-25 , DOI: 10.1002/adsc.202000884 Annapurna Awasthi 1, 2 , Pushpendra Yadav 1, 2 , Virendra Kumar 3 , Dharmendra Kumar Tiwari 1
Affiliation
|
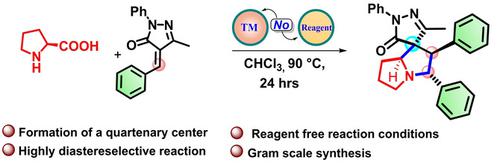
An efficient and reagent‐free synthesis of highly functionalized aza‐spirocyclic pyrazolones are achieved from easily available α‐amino acids and alkylidene pyrazolones by means of amination, C−C‐double bonds cleavage, and decarboxylative annulation process. These highly diastereoselective reactions are promoted simply by α‐amino acids and involve in situ generated azomethine ylides as reactive intermediates. This newly developed protocol involves the formation of three new bonds (one C−N and two C−C) and four new contiguous stereo‐centers including a quaternary carbon center in a single pot cascade process.
中文翻译:

α-氨基酸介导的C-C双键裂解在Aza-螺环吡唑啉酮的非对映选择性合成中的作用
通过胺化,CC双键裂解和脱羧环化工艺,可以轻松获得α-氨基酸和亚烷基吡唑啉酮,从而高效高效地合成高度官能化的氮杂-螺环吡唑啉酮。这些高度非对映选择性反应仅通过α-氨基酸促进,并涉及原位生成的偶氮甲亚胺作为反应性中间体。这项新开发的协议涉及在单个锅级联过程中形成三个新键(一个C N和两个C C)和四个新的连续立体中心,包括一个季碳中心。
更新日期:2020-10-26
中文翻译:

α-氨基酸介导的C-C双键裂解在Aza-螺环吡唑啉酮的非对映选择性合成中的作用
通过胺化,CC双键裂解和脱羧环化工艺,可以轻松获得α-氨基酸和亚烷基吡唑啉酮,从而高效高效地合成高度官能化的氮杂-螺环吡唑啉酮。这些高度非对映选择性反应仅通过α-氨基酸促进,并涉及原位生成的偶氮甲亚胺作为反应性中间体。这项新开发的协议涉及在单个锅级联过程中形成三个新键(一个C N和两个C C)和四个新的连续立体中心,包括一个季碳中心。











































 京公网安备 11010802027423号
京公网安备 11010802027423号