当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of 1,4‐Dicarbonyl Compounds by Visible‐Light‐Mediated Cross‐Coupling Reactions of α‐Chlorocarbonyls and Enol Acetates
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-08-25 , DOI: 10.1002/adsc.202000791 Qiang Liu 1 , Rui‐Guo Wang 1 , Hong‐Jian Song 1 , Yu‐Xiu Liu 1 , Qing‐Min Wang 1, 2
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-08-25 , DOI: 10.1002/adsc.202000791 Qiang Liu 1 , Rui‐Guo Wang 1 , Hong‐Jian Song 1 , Yu‐Xiu Liu 1 , Qing‐Min Wang 1, 2
Affiliation
|
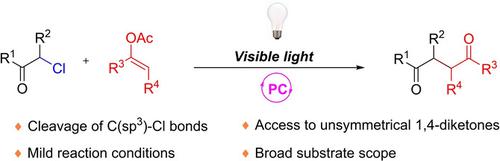
Herein, we report a protocol for visible‐light‐mediated radical coupling reactions of α‐chloroketones and enol acetates to afford 1,4‐dicarbonyl compounds, which are important precursors and intermediates in organic synthesis. The reaction involves photoredox‐catalyzed activation of the α‐chloroketone upon photoelectron transfer, carbon–chlorine bond cleavage, and coupling of the resulting radical with the carbon–carbon double bond of the enol acetate. This mild protocol has a wide substrate scope and moderate to good yields.
中文翻译:

α-氯代羰基化合物与烯醇式乙酸酯的可见光介导的交叉偶联反应合成1,4-二羰基化合物
在此,我们报告了一种协议,用于α-氯酮和烯醇乙酸酯的可见光介导的自由基偶联反应,以提供1,4-二羰基化合物,它们是有机合成中的重要前体和中间体。该反应涉及光电子转移,碳-氯键裂解以及光自由基与烯醇乙酸酯的碳-碳双键的偶联被α-氯酮的光氧化还原催化活化。这种温和的方案具有广泛的底物范围和中等至良好的产量。
更新日期:2020-10-26
中文翻译:

α-氯代羰基化合物与烯醇式乙酸酯的可见光介导的交叉偶联反应合成1,4-二羰基化合物
在此,我们报告了一种协议,用于α-氯酮和烯醇乙酸酯的可见光介导的自由基偶联反应,以提供1,4-二羰基化合物,它们是有机合成中的重要前体和中间体。该反应涉及光电子转移,碳-氯键裂解以及光自由基与烯醇乙酸酯的碳-碳双键的偶联被α-氯酮的光氧化还原催化活化。这种温和的方案具有广泛的底物范围和中等至良好的产量。











































 京公网安备 11010802027423号
京公网安备 11010802027423号