当前位置:
X-MOL 学术
›
Biotechnol. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Self-Assembled Peptide-Based Biocomposites for Near-Infrared Light Triggered Drug Release to Tumor Cells.
Biotechnology Journal ( IF 3.2 ) Pub Date : 2020-08-26 , DOI: 10.1002/biot.202000128 Rachel E Daso 1 , Ipsita A Banerjee 1
Biotechnology Journal ( IF 3.2 ) Pub Date : 2020-08-26 , DOI: 10.1002/biot.202000128 Rachel E Daso 1 , Ipsita A Banerjee 1
Affiliation
|
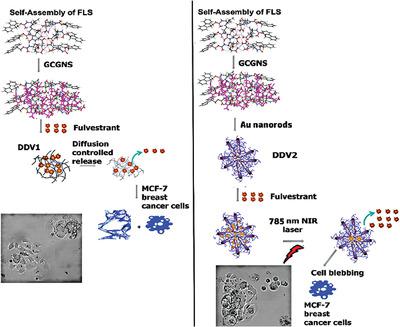
Peptide‐based nanomaterials are increasingly gaining popularity due to their specificity, biocompatibility, and biodegradability. In this work, a new multi‐layered peptide‐based biocomposite for targeting MCF‐7 breast cancer cells is developed. The amphipathic Fluorenylmethyloxycarbonyl (Fmoc)‐Leu‐Ser peptide is synthesized, which is conjugated to a tumor‐targeting peptide sequence Gly‐Cys‐Gly‐Asn‐Ser to form Fmoc‐L‐S‐G‐C‐G‐N‐S (FLS) assemblies. To the FLS assemblies, gold nanorods are then attached to develop drug delivery vehicles (DDVs). The DDVs are entrapped with the anti‐cancer drug fulvestrant. Entrapment efficiency is found to be 50.6%. Release studies indicate that irradiating the gold nanorod bound DDVs at NIR wavelength (785 nm) increases drug release by fourfold compared to assemblies that are not irradiated. These results also show higher cytotoxicity and lower cell invasion due to photo‐triggered drug release. Furthermore, distinct actin cytoskeletal changes are observed. Such novel peptide‐based gold nanorod bound DDVs demonstrate potential in dual targeting of MCF‐7 breast cancer cells.
中文翻译:

自组装的基于肽的近红外生物复合材料触发药物释放到肿瘤细胞。
基于肽的纳米材料由于其特异性,生物相容性和可生物降解性而越来越受欢迎。在这项工作中,开发了一种针对MCF-7乳腺癌细胞的新型多层基于肽的生物复合材料。合成两亲性芴基甲氧基羰基(Fmoc)‐Leu‐Ser肽,将其与靶向肿瘤的肽序列Gly‐Cys‐Gly‐Asn‐Ser偶联形成Fmoc‐L‐S‐G‐C‐G‐N‐S (FLS)程序集。然后,将金纳米棒连接到FLS组件,以开发药物输送工具(DDV)。DDV含有抗癌药物氟维司群。发现包封率是50.6%。释放研究表明,与未辐照的组件相比,在NIR波长(785 nm)处辐照结合金纳米棒的DDV可使药物释放增加四倍。这些结果还表明,由于光触发药物释放,具有更高的细胞毒性和更低的细胞侵袭性。此外,观察到明显的肌动蛋白细胞骨架变化。这种新型的基于肽的金纳米棒结合的DDV展示了MCF-7乳腺癌细胞双重靶向的潜力。
更新日期:2020-08-26
中文翻译:

自组装的基于肽的近红外生物复合材料触发药物释放到肿瘤细胞。
基于肽的纳米材料由于其特异性,生物相容性和可生物降解性而越来越受欢迎。在这项工作中,开发了一种针对MCF-7乳腺癌细胞的新型多层基于肽的生物复合材料。合成两亲性芴基甲氧基羰基(Fmoc)‐Leu‐Ser肽,将其与靶向肿瘤的肽序列Gly‐Cys‐Gly‐Asn‐Ser偶联形成Fmoc‐L‐S‐G‐C‐G‐N‐S (FLS)程序集。然后,将金纳米棒连接到FLS组件,以开发药物输送工具(DDV)。DDV含有抗癌药物氟维司群。发现包封率是50.6%。释放研究表明,与未辐照的组件相比,在NIR波长(785 nm)处辐照结合金纳米棒的DDV可使药物释放增加四倍。这些结果还表明,由于光触发药物释放,具有更高的细胞毒性和更低的细胞侵袭性。此外,观察到明显的肌动蛋白细胞骨架变化。这种新型的基于肽的金纳米棒结合的DDV展示了MCF-7乳腺癌细胞双重靶向的潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号