当前位置:
X-MOL 学术
›
Int. J. Quantum Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A density functional theory study on the structure formation of Al(III) carboxylate complexes in aqueous aluminum sols
International Journal of Quantum Chemistry ( IF 2.3 ) Pub Date : 2020-08-25 , DOI: 10.1002/qua.26430 Chunlan Li 1 , Wensheng Liu 1 , Juan Wang 1 , Shuwei Yao 1 , Yunzhu Ma 1
International Journal of Quantum Chemistry ( IF 2.3 ) Pub Date : 2020-08-25 , DOI: 10.1002/qua.26430 Chunlan Li 1 , Wensheng Liu 1 , Juan Wang 1 , Shuwei Yao 1 , Yunzhu Ma 1
Affiliation
|
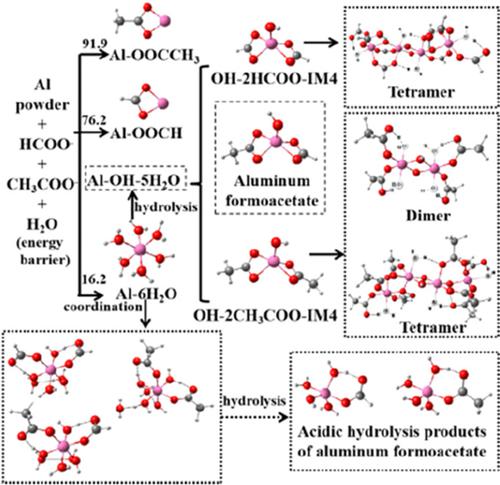
In‐depth studies of the structure of precursor sols is very important for understanding the subsequent sol to gel transition. Here, density functional theory was applied to study the reaction of Al powder with formic and acetic acid in aqueous solution to find the structure of Al(III) complex in precursor sols. The Al3+ ion obtained by dissolving Al powder in aqueous solution of formic and acetic acid would preferentially coordinate with water molecules to form aluminum hydrates. As the reaction energy barrier of aluminum hydrates with CH3COO− and HCOO− was comparable, both aluminum formoacetate and other aluminum carboxylate monomers could be obtained. Most Al(III) complexes formed by other carboxylate monomers were similar to that prepared from aluminum formoacetate. However, some Al(III) monomers and Al(III) dimer with different structural characteristics might be detrimental to the formation of linear polymers. The formation of other aluminum carboxylate monomers had great influence on the structure of precursor sols.
中文翻译:

水性铝溶胶中羧酸铝(III)配合物结构形成的密度泛函理论研究
深入研究前体溶胶的结构对于理解随后的溶胶向凝胶的转变非常重要。在这里,应用密度泛函理论研究了铝粉与甲酸和乙酸在水溶液中的反应,以发现前体溶胶中的Al(III)配合物的结构。通过将Al粉溶解在甲酸和乙酸的水溶液中而获得的Al 3+离子将优先与水分子配位形成水合铝。如用CH铝水合物的反应能垒3 COO -和HCOO -与之相当,可以得到甲酸铝和其他羧酸铝单体。由其他羧酸盐单体形成的大多数Al(III)络合物与由甲酸乙酸铝制备的络合物相似。但是,某些具有不同结构特征的Al(III)单体和Al(III)二聚体可能不利于线性聚合物的形成。其他羧酸铝单体的形成对前体溶胶的结构影响很大。
更新日期:2020-08-25
中文翻译:

水性铝溶胶中羧酸铝(III)配合物结构形成的密度泛函理论研究
深入研究前体溶胶的结构对于理解随后的溶胶向凝胶的转变非常重要。在这里,应用密度泛函理论研究了铝粉与甲酸和乙酸在水溶液中的反应,以发现前体溶胶中的Al(III)配合物的结构。通过将Al粉溶解在甲酸和乙酸的水溶液中而获得的Al 3+离子将优先与水分子配位形成水合铝。如用CH铝水合物的反应能垒3 COO -和HCOO -与之相当,可以得到甲酸铝和其他羧酸铝单体。由其他羧酸盐单体形成的大多数Al(III)络合物与由甲酸乙酸铝制备的络合物相似。但是,某些具有不同结构特征的Al(III)单体和Al(III)二聚体可能不利于线性聚合物的形成。其他羧酸铝单体的形成对前体溶胶的结构影响很大。











































 京公网安备 11010802027423号
京公网安备 11010802027423号