当前位置:
X-MOL 学术
›
Chem. Asian J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Anomalously Slow Conformational Change Dynamics of Polar Groups Anchored to Hydrophobic Surfaces in Aqueous Media.
Chemistry - An Asian Journal ( IF 3.5 ) Pub Date : 2020-08-26 , DOI: 10.1002/asia.202000742 Tengfei Fu 1 , Hao Xing 1, 2 , Eric S Silver 1 , Yoshimitsu Itoh 1 , Shuo Chen 1 , Takuya Masuda 3, 4 , Kohei Uosaki 4, 5 , Feihe Huang 2 , Takuzo Aida 1, 6
Chemistry - An Asian Journal ( IF 3.5 ) Pub Date : 2020-08-26 , DOI: 10.1002/asia.202000742 Tengfei Fu 1 , Hao Xing 1, 2 , Eric S Silver 1 , Yoshimitsu Itoh 1 , Shuo Chen 1 , Takuya Masuda 3, 4 , Kohei Uosaki 4, 5 , Feihe Huang 2 , Takuzo Aida 1, 6
Affiliation
|
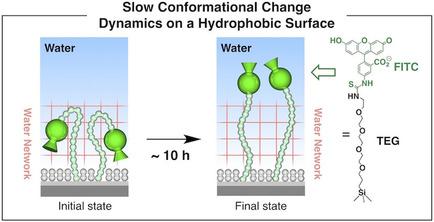
Water molecules within a thin hydration layer, spontaneously generated on hydrophobic protein surfaces, are reported to form a poorly dynamic network structure. However, how such a water network affects the conformational change dynamics of polar groups has never been explored, although such polar groups play a critical role in protein‐protein and protein‐ligand interactions. In the present work, we utilized as model protein surfaces a series of self‐assembled monolayers (SAMs) appended with polar (Fmoc) or ionic (FITC) fluorescent head groups that were tethered via a 1.5‐nm‐long flexible oligoether chain to a hydrophobic silicon wafer surface, which was densely covered with paraffinic chains. We found that, not only in deionized water but also in aqueous buffer, these oligoether‐appended head groups at ambient temperatures both displayed an anomalously slow conformational change, which required ∼10 h to reach a thermodynamically equilibrated state. We suppose that these behaviors reflect the poorly dynamic and low‐permittivity natures of the thin hydration layer.
中文翻译:

固定在水性介质中疏水表面上的极性基团异常缓慢的构象变化动力学。
据报道,在疏水蛋白表面上自发产生的薄水化层中的水分子形成了动态性较差的网络结构。然而,尽管这种极性基团在蛋白质-蛋白质和蛋白质-配体相互作用中起着关键作用,但从未探讨过这种水网络如何影响极性基团的构象变化动力学。在当前的工作中,我们利用一系列自组装的单分子层(SAMs)作为模型蛋白质表面,并附加了通过束缚的极性(Fmoc)或离子(FITC)荧光头基一条1.5纳米长的柔性寡醚链连接到疏水的硅晶片表面,该表面被石蜡链密集地覆盖。我们发现,不仅在去离子水中,而且在水性缓冲液中,这些在环境温度下低聚醚的头部均显示出异常缓慢的构象变化,这需要约10小时才能达到热力学平衡状态。我们认为这些行为反映了薄水化层的不良动态和低介电常数性质。
更新日期:2020-10-17
中文翻译:

固定在水性介质中疏水表面上的极性基团异常缓慢的构象变化动力学。
据报道,在疏水蛋白表面上自发产生的薄水化层中的水分子形成了动态性较差的网络结构。然而,尽管这种极性基团在蛋白质-蛋白质和蛋白质-配体相互作用中起着关键作用,但从未探讨过这种水网络如何影响极性基团的构象变化动力学。在当前的工作中,我们利用一系列自组装的单分子层(SAMs)作为模型蛋白质表面,并附加了通过束缚的极性(Fmoc)或离子(FITC)荧光头基一条1.5纳米长的柔性寡醚链连接到疏水的硅晶片表面,该表面被石蜡链密集地覆盖。我们发现,不仅在去离子水中,而且在水性缓冲液中,这些在环境温度下低聚醚的头部均显示出异常缓慢的构象变化,这需要约10小时才能达到热力学平衡状态。我们认为这些行为反映了薄水化层的不良动态和低介电常数性质。









































 京公网安备 11010802027423号
京公网安备 11010802027423号