当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Diastereoselective Synthesis of Spiro[indoline‐3,7′‐pyrrolo[1,2‐a]azepines] via Sequential [3+2] Cycloaddition and Ring Expansion Reaction
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2020-08-26 , DOI: 10.1002/ajoc.202000386 Wen‐Tao Wu 1 , Ying Han 1 , Jing Sun 1 , Chao‐Guo Yan 1
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2020-08-26 , DOI: 10.1002/ajoc.202000386 Wen‐Tao Wu 1 , Ying Han 1 , Jing Sun 1 , Chao‐Guo Yan 1
Affiliation
|
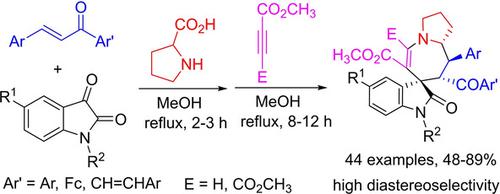
Spiro[indoline‐3,7′‐pyrrolo[1,2‐a]azepines] were conveniently synthesized in satisfactory yields and with high diastereoselectivity from three‐component reaction of L‐proline, isatins and chalcones and sequential reaction with dimethyl but‐2‐ynedioate or methyl propiolate in refluxing methanol. The ferrocenyl‐chalcones and dibenzylideneacetones were also successfully used in this one‐pot two‐step reaction to give novel ferrocenyl and styryl‐substituted spiro compounds. The reaction mechanism included domino [3+2] cycloadddtition reaction of azomethine ylide with chalcone and ring‐expansion reaction of in situ generated spiro[indoline‐3,3′‐pyrrolizine] with electron‐deficient alkynes.
中文翻译:

[3 + 2]环加成和扩环反应非对映选择性合成螺[吲哚啉-3,7'-吡咯并[1,2-a]氮杂]
螺[吲哚啉-3,7'-吡咯并[1,2- a ]氮杂]可以方便地以令人满意的收率合成,并具有高的非对映选择性,这是由L-脯氨酸,靛红和查耳酮的三组分反应以及与二甲基丁-2-的顺序反应回流的甲醇中的乙炔或丙酸甲酯。二茂铁基-查耳酮和二亚苄基丙酮也成功用于此一锅两步反应,得到了新的二茂铁基和苯乙烯基取代的螺环化合物。反应机理包括偶氮甲meth内酯与查耳酮的多米诺[3 + 2]环加成反应以及原位生成的螺[吲哚啉-3,3'-吡咯嗪]与缺电子炔烃的扩环反应。
更新日期:2020-08-26
中文翻译:

[3 + 2]环加成和扩环反应非对映选择性合成螺[吲哚啉-3,7'-吡咯并[1,2-a]氮杂]
螺[吲哚啉-3,7'-吡咯并[1,2- a ]氮杂]可以方便地以令人满意的收率合成,并具有高的非对映选择性,这是由L-脯氨酸,靛红和查耳酮的三组分反应以及与二甲基丁-2-的顺序反应回流的甲醇中的乙炔或丙酸甲酯。二茂铁基-查耳酮和二亚苄基丙酮也成功用于此一锅两步反应,得到了新的二茂铁基和苯乙烯基取代的螺环化合物。反应机理包括偶氮甲meth内酯与查耳酮的多米诺[3 + 2]环加成反应以及原位生成的螺[吲哚啉-3,3'-吡咯嗪]与缺电子炔烃的扩环反应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号