当前位置:
X-MOL 学术
›
Tetrahedron Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Intramolecular asymmetric oxidopyrylium-based [5 + 2] cycloadditions
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2020-08-26 , DOI: 10.1016/j.tetlet.2020.152377 Samantha N. Rokey , Justin A. Simanis , Chunyin M. Law , Shilpa Pohani , Samantha Willens Behrends , Jacob J. Bulandr , Gregory M. Ferrence , John R. Goodell , T. Andrew Mitchell
中文翻译:

分子内不对称基于氧化吡啶鎓的[5 + 2]环加成
更新日期:2020-09-20
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2020-08-26 , DOI: 10.1016/j.tetlet.2020.152377 Samantha N. Rokey , Justin A. Simanis , Chunyin M. Law , Shilpa Pohani , Samantha Willens Behrends , Jacob J. Bulandr , Gregory M. Ferrence , John R. Goodell , T. Andrew Mitchell
|
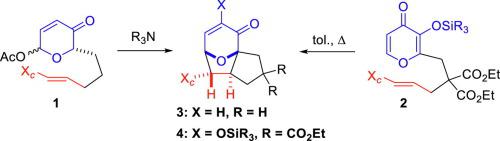
Intramolecular oxidopyrylium-based [5 + 2] cycloadditions utilizing chiral auxiliaries were investigated. Both acetoxypyranones and silyloxypyrones were employed and sulfinimines, oxazolidinones, hydrazones, and chiral enamines were explored. Carbonyl-based auxiliaries gave low selectivity, but enamines afforded excellent diastereoselectivity. Overall, facial selectivity varied significantly providing insight regarding the scope and limitation of chiral auxiliary-based oxidopyrylium-alkene [5 + 2] cycloadditions.
中文翻译:

分子内不对称基于氧化吡啶鎓的[5 + 2]环加成
利用手性助剂研究了基于分子内氧化吡啶基的[5 + 2]环加成反应。乙酰氧基吡喃酮和甲硅烷氧基吡喃酮均被使用,并研究了亚磺胺,恶唑烷酮,和手性烯胺。基于羰基的助剂具有较低的选择性,但烯胺具有出色的非对映选择性。总体而言,面部选择性变化很大,从而提供了有关基于手性助剂的氧化吡啶烯-烯烃[5 + 2]环加成反应的范围和限制的见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号