Tetrahedron Letters ( IF 1.5 ) Pub Date : 2020-08-26 , DOI: 10.1016/j.tetlet.2020.152382 Soumya Dutta , Amit Saha
|
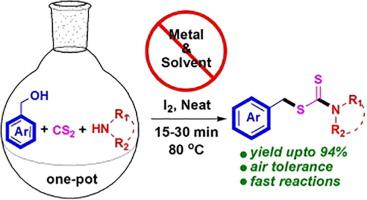
An efficient, metal and solvent free synthesis of S-benzylic dithiocarbamate esters has been demonstrated via the iodine mediated direct C-S coupling of benzylic alcohols with dithiocarbamate anions generated in-situ by the reactions of amines and carbon disulphide. All the reactions were very fast (15–30 min) and performed under open air atmosphere. Cyclic and acyclic secondary amines, primary amine, aromatic amine actively participated in the one-pot coupling reactions with different benzylic alcohols. Non benzylic alcohols offer the synthesis of O-thiocarbamate compounds under the identical reaction condition.
中文翻译:

碘介导的苄醇与二硫代氨基甲酸酯阴离子的直接偶联:在纯净的反应条件下,S-苄基二硫代氨基甲酸酯的简便连接
通过碘介导的苄醇与由胺和二硫化碳反应原位产生的二硫代氨基甲酸酯阴离子的直接介导的CS偶联,已经证明了S-苄基的二硫代氨基甲酸酯的有效,无金属和溶剂的合成。所有反应都非常快(15-30分钟),并且在露天气氛下进行。环状和无环仲胺,伯胺,芳族胺积极参与与不同苯甲醇的一锅偶联反应。非苄醇在相同的反应条件下提供邻硫代氨基甲酸酯化合物的合成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号