Molecular Cell ( IF 14.5 ) Pub Date : 2020-08-26 , DOI: 10.1016/j.molcel.2020.08.003 Hannah K Reinking 1 , Hyun-Seo Kang 2 , Maximilian J Götz 1 , Hao-Yi Li 1 , Anja Kieser 1 , Shubo Zhao 1 , Aleida C Acampora 1 , Pedro Weickert 1 , Evelyn Fessler 1 , Lucas T Jae 1 , Michael Sattler 2 , Julian Stingele 1
|
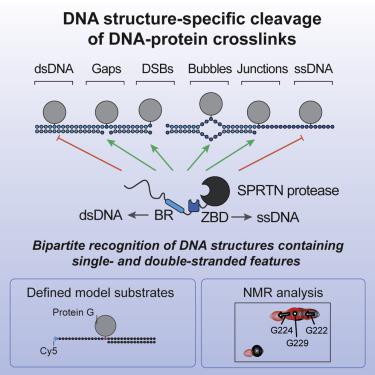
Repair of covalent DNA-protein crosslinks (DPCs) by DNA-dependent proteases has emerged as an essential genome maintenance mechanism required for cellular viability and tumor suppression. However, how proteolysis is restricted to the crosslinked protein while leaving surrounding chromatin proteins unharmed has remained unknown. Using defined DPC model substrates, we show that the DPC protease SPRTN displays strict DNA structure-specific activity. Strikingly, SPRTN cleaves DPCs at or in direct proximity to disruptions within double-stranded DNA. In contrast, proteins crosslinked to intact double- or single-stranded DNA are not cleaved by SPRTN. NMR spectroscopy data suggest that specificity is not merely affinity-driven but achieved through a flexible bipartite strategy based on two DNA binding interfaces recognizing distinct structural features. This couples DNA context to activation of the enzyme, tightly confining SPRTN’s action to biologically relevant scenarios.
中文翻译:

SPRTN蛋白酶对DNA蛋白交联键的DNA结构特异性切割。
DNA依赖性蛋白酶修复共价DNA-蛋白质交联键(DPC)已成为细胞生存力和肿瘤抑制所需的重要基因组维持机制。然而,如何将蛋白水解限制在交联蛋白上而又不破坏周围的染色质蛋白仍是未知的。使用定义的DPC模型底物,我们显示DPC蛋白酶SPRTN显示严格的DNA结构特异性活性。令人惊讶的是,SPRTN在双链DNA内的断裂处或紧邻断裂处切割DPC。相反,与完整的双链或单链DNA交联的蛋白质不会被SPRTN切割。NMR光谱数据表明,特异性不仅是亲和力驱动的,而且是通过基于两个识别不同结构特征的DNA结合界面的灵活二分策略实现的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号