Journal of Molecular Biology ( IF 4.7 ) Pub Date : 2020-08-26 , DOI: 10.1016/j.jmb.2020.08.017 Eliane H Yardeni 1 , Smriti Mishra 2 , Richard A Stein 2 , Eitan Bibi 1 , Hassane S Mchaourab 2
|
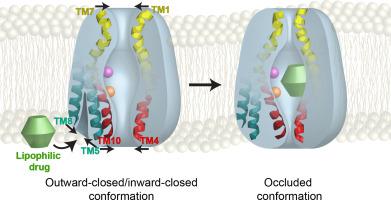
The prototypic multidrug (Mdr) transporter MdfA from Escherichia coli efflux chemically‐ dissimilar substrates in exchange for protons. Similar to other transporters, MdfA purportedly functions by alternating access of a central substrate binding pocket to either side of the membrane. Accordingly, MdfA should open at the cytoplasmic side and/or laterally toward the membrane to enable access of drugs into its pocket. At the end of the cycle, the periplasmic side is expected to open to release drugs. Two distinct conformations of MdfA have been captured by X-ray crystallography: An outward open (Oo) conformation, stabilized by a Fab fragment, and a ligand-bound inward-facing (If) conformation, possibly stabilized by a mutation (Q131R). Here, we investigated how these structures relate to ligand-dependent conformational dynamics of MdfA in lipid bilayers. For this purpose, we combined distances measured by double electron-electron resonance (DEER) between pairs of spin labels in MdfA, reconstituted in nanodiscs, with cysteine cross-linking of natively expressed membrane-embedded MdfA variants. Our results suggest that in a membrane environment, MdfA assumes a relatively flexible, outward-closed/inward-closed (Oc/Ic) conformation. Unexpectedly, our data show that neither the substrate TPP nor protonation induces large-scale conformational changes. Rather, we identified a substrate-responsive lateral gate, which is open toward the inner leaflet of the membrane but closes upon drug binding. Together, our results suggest a modified model for the functional conformational cycle of MdfA that does not invoke canonical elements of alternating access.
中文翻译:

多药转运蛋白 MdfA 偏离了 MFS 转运蛋白交替通路的规范模型。
来自大肠杆菌的原型多药 (Mdr) 转运蛋白 MdfA流出化学性质不同的底物以交换质子。与其他转运蛋白类似,据称 MdfA 的功能是通过交替进入膜两侧的中央底物结合口袋。因此,MdfA 应该在细胞质侧和/或横向向膜开放,以使药物能够进入其口袋。在周期结束时,周质侧有望打开以释放药物。X 射线晶体学已捕获 MdfA 的两种不同构象:向外开放 (O o ) 构象,由 Fab 片段稳定,以及配体结合的向内 (I f) 构象,可能通过突变 (Q131R) 稳定。在这里,我们研究了这些结构如何与脂质双层中 MdfA 的配体依赖性构象动力学相关。为此,我们将 MdfA 中成对自旋标记之间的双电子-电子共振 (DEER) 测量的距离结合起来,在纳米圆盘中重建,与天然表达的膜嵌入 MdfA 变体的半胱氨酸交联。我们的结果表明,在膜环境中,MdfA 假定一个相对灵活的、向外封闭/向内封闭的 (O c /I c) 构象。出乎意料的是,我们的数据显示底物 TPP 和质子化都不会引起大规模的构象变化。相反,我们确定了一个底物响应侧门,它向膜的内小叶开放,但在药物结合时关闭。总之,我们的结果提出了一种修改后的 MdfA 功能构象循环模型,该模型不会调用交替访问的规范元素。











































 京公网安备 11010802027423号
京公网安备 11010802027423号