Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2020-08-26 , DOI: 10.1016/j.bioorg.2020.104190 Lidija-Marija Tumir 1 , Iva Zonjić 1 , Kristina Žuna 2 , Sandra Radić Brkanac 3 , Marijana Jukić 4 , Ana Huđek 2 , Ksenija Durgo 2 , Ivo Crnolatac 1 , Ljubica Glavaš-Obrovac 4 , Nunzio Cardullo 5 , Luana Pulvirenti 5 , Vera Muccilli 5 , Corrado Tringali 5 , Marijana Radić Stojković 1
|
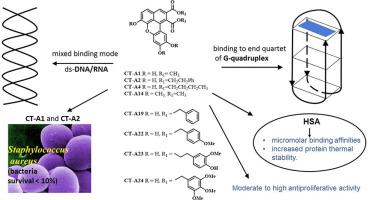
Interactions of two newly synthesized and six previously reported benzoxanthene lignans (BXLs), analogues of rare natural products, with DNA/RNA, G-quadruplex and HSA were evaluated by a set of spectrophotometric methods. Presence/absence of methoxy and hydroxy groups on the benzoxanthene core and minor modifications at C-1/C-2 side pendants – presence/absence of phenyl ring and presence/absence of methoxy and hydroxy groups on phenyl ring – influenced the fluorescence changes and the binding strength to double-stranded (ds-) and G-quadruplex structures. In general, compounds without phenyl ring showed stronger fluorescence changes upon binding than phenyl-substituted BXLs. On the other hand, BXLs with an unsubstituted phenyl ring showed the best stabilization effects of G-quadruplex. Circular dichroism spectroscopy results suggest mixed binding mode, groove binding and partial intercalation, to ds-DNA/RNA and end-stacking to top or bottom G-tetrads as the main binding modes of BXLs to those targets. All compounds exhibited micromolar binding affinities toward HSA and an increased protein thermal stability. Moderate to strong antiradical scavenging activity was observed for all BXLs with hydroxy groups at C-6, C-9 and C-10 positions of the benzoxanthene core, except for derivative bearing methoxy groups at these positions. BXLs with unsubstituted or low-substituted phenyl ring and one derivative without phenyl ring showed strong growth inhibition of Gram-positive Staphylococcus aureus. All compounds showed moderate to strong tumor cell growth-inhibitory activity and cytotoxicity.
中文翻译:

苯并[k,l] x吨木脂素的合成,DNA / RNA相互作用和生物活性。
通过一套分光光度法评估了两种新合成的和六种先前报道的苯并氧杂蒽木脂素(BXL)(稀有天然产物的类似物)与DNA / RNA,G-四链体和HSA的相互作用。苯并氧杂蒽核心上是否存在甲氧基和羟基,以及C-1 / C-2侧挂基上的微小修饰-苯环是否存在以及苯环上是否存在甲氧基和羟基-影响了荧光变化,对双链(ds-)和G-四链体结构的结合强度。通常,不具有苯环的化合物在结合时比苯基取代的BXL表现出更强的荧光变化。另一方面,具有未取代的苯环的BXL表现出G-四链体的最佳稳定效果。圆二色光谱结果表明,与ds-DNA / RNA的混合结合方式,凹槽结合和部分嵌入,以及最终堆叠至顶部或底部G-四联体,是BXL与这些靶标的主要结合方式。所有化合物均表现出对HSA的微摩尔结合亲和力和增加的蛋白质热稳定性。对于在苯并氧杂蒽核的C-6,C-9和C-10位置带有羟基的所有BXL,均观察到了中等至强的抗自由基清除活性,但在这些位置带有甲氧基的衍生物除外。具有未取代或低取代的苯环和一种不具有苯环的衍生物的BXL表现出对革兰氏阳性细胞的强烈抑制作用 所有化合物均表现出对HSA的微摩尔结合亲和力和增加的蛋白质热稳定性。对于在苯并氧杂蒽核的C-6,C-9和C-10位置带有羟基的所有BXL,均观察到了中等至强的抗自由基清除活性,但在这些位置带有甲氧基的衍生物除外。具有未取代或低取代的苯环和一种不具有苯环的衍生物的BXL表现出对革兰氏阳性细胞的强烈抑制作用 所有化合物均表现出对HSA的微摩尔结合亲和力和增加的蛋白质热稳定性。对于在苯并氧杂蒽核心的C-6,C-9和C-10位置带有羟基的所有BXL,均观察到了中等至强的抗自由基清除活性,但在这些位置带有甲氧基的衍生物除外。具有未取代或低取代苯环和一种不具有苯环的衍生物的BXL表现出对革兰氏阳性细胞的强烈抑制作用金黄色葡萄球菌。所有化合物均表现出中等至强的肿瘤细胞生长抑制活性和细胞毒性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号