当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Functional and Structural Insights into a Novel Promiscuous Ketoreductase of the Lugdunomycin Biosynthetic Pathway.
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2020-08-25 , DOI: 10.1021/acschembio.0c00564 Xiansha Xiao 1 , Somayah S Elsayed 1 , Changsheng Wu 2 , Helga U van der Heul 1 , Mikko Metsä-Ketelä 3 , Chao Du 1 , Andrea E Prota 4 , Chun-Chi Chen 5 , Weidong Liu 5 , Rey-Ting Guo 5 , Jan Pieter Abrahams 1, 6, 7 , Gilles P van Wezel 1
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2020-08-25 , DOI: 10.1021/acschembio.0c00564 Xiansha Xiao 1 , Somayah S Elsayed 1 , Changsheng Wu 2 , Helga U van der Heul 1 , Mikko Metsä-Ketelä 3 , Chao Du 1 , Andrea E Prota 4 , Chun-Chi Chen 5 , Weidong Liu 5 , Rey-Ting Guo 5 , Jan Pieter Abrahams 1, 6, 7 , Gilles P van Wezel 1
Affiliation
|
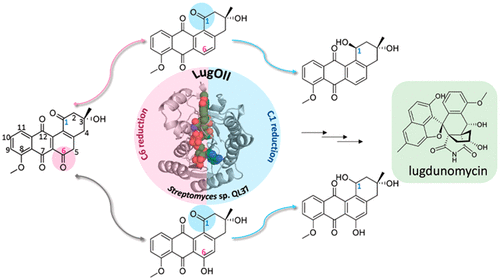
Angucyclines are a structurally diverse class of actinobacterial natural products defined by their varied polycyclic ring systems, which display a wide range of biological activities. We recently discovered lugdunomycin (1), a highly rearranged polyketide antibiotic derived from the angucycline backbone that is synthesized via several yet unexplained enzymatic reactions. Here, we show via in vivo, in vitro, and structural analysis that the promiscuous reductase LugOII catalyzes both a C6 and an unprecedented C1 ketoreduction. This then sets the stage for the subsequent C-ring cleavage that is key to the rearranged scaffolds of 1. The 1.1 Å structures of LugOII in complex with either ligand 8-O-Methylrabelomycin (4) or 8-O-Methyltetrangomycin (5) and of apoenzyme were resolved, which revealed a canonical Rossman fold and a remarkable conformational change during substrate capture and release. Mutational analysis uncovered key residues for substrate access, position, and catalysis as well as specific determinants that control its dual functionality. The insights obtained in this work hold promise for the discovery and engineering of other promiscuous reductases that may be harnessed for the generation of novel biocatalysts for chemoenzymatic applications.
中文翻译:

功能和结构的见解,为Lugdunomycin生物合成途径的新型混杂的酮还原酶。
安古环素是一类结构多样的放线菌天然产物,由其各种多环系统定义,它们具有广泛的生物活性。我们最近发现了lugdunomycin(1),这是一种高度重排的聚酮类抗生素,它是从安古环素主链衍生而来的,它是通过几种尚未解释的酶促反应合成的。在这里,我们通过体内,体外和结构分析表明,混杂的还原酶LugOII催化C6和前所未有的C1酮还原。然后,这为随后的C环裂解奠定了基础,这对1的重排支架至关重要。带有配体8- O的LugOII的1.1Å结构解决了-甲基雷贝霉素(4)或8- O-甲基四霉素(5)和脱辅酶的问题,这揭示了典型的Rossman折叠以及底物捕获和释放过程中显着的构象变化。突变分析发现了底物进入,位置和催化的关键残基,以及控制其双重功能的特定决定簇。在这项工作中获得的见识为发现和工程设计其他混杂还原酶提供了希望,这些杂合还原酶可用于产生化学酶应用的新型生物催化剂。
更新日期:2020-09-20
中文翻译:

功能和结构的见解,为Lugdunomycin生物合成途径的新型混杂的酮还原酶。
安古环素是一类结构多样的放线菌天然产物,由其各种多环系统定义,它们具有广泛的生物活性。我们最近发现了lugdunomycin(1),这是一种高度重排的聚酮类抗生素,它是从安古环素主链衍生而来的,它是通过几种尚未解释的酶促反应合成的。在这里,我们通过体内,体外和结构分析表明,混杂的还原酶LugOII催化C6和前所未有的C1酮还原。然后,这为随后的C环裂解奠定了基础,这对1的重排支架至关重要。带有配体8- O的LugOII的1.1Å结构解决了-甲基雷贝霉素(4)或8- O-甲基四霉素(5)和脱辅酶的问题,这揭示了典型的Rossman折叠以及底物捕获和释放过程中显着的构象变化。突变分析发现了底物进入,位置和催化的关键残基,以及控制其双重功能的特定决定簇。在这项工作中获得的见识为发现和工程设计其他混杂还原酶提供了希望,这些杂合还原酶可用于产生化学酶应用的新型生物催化剂。









































 京公网安备 11010802027423号
京公网安备 11010802027423号