当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Palladium-Catalyzed Intermolecular Trans-Selective Carbofunctionalization of Internal Alkynes to Highly Functionalized Alkenes
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-08-24 , DOI: 10.1021/acscatal.0c02522 Weiwei Lv 1 , Shihan Liu 2 , Yanhui Chen 1 , Si Wen 1 , Yu Lan 2, 3 , Guolin Cheng 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-08-24 , DOI: 10.1021/acscatal.0c02522 Weiwei Lv 1 , Shihan Liu 2 , Yanhui Chen 1 , Si Wen 1 , Yu Lan 2, 3 , Guolin Cheng 1
Affiliation
|
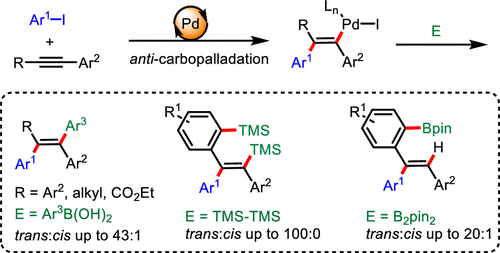
A palladium/DPEphos-catalyzed intermolecular trans-selective carbofunctionalization of internal alkynes has been established herein. This method proceeds through a formal anti-carbopalladation, forming trans-alkenyl palladium species, which was trapped by aryl boronic acids to provide all-carbon tetrasubstituted alkenes in 32–92% yields. The trans-selective arylsilylation/remote C–H silylation and hydroarylation/remote C–H borylation of internal alkynes were also achieved using hexamethyldisilane and bis(pinacolato)diboron as trapping reagents, respectively. The reaction features good regio- and stereoselectivity and high functional group tolerance. A preliminary mechanistic study and DFT calculations show that a cis to trans isomerization of cis-alkenyl palladium species was involved in this transformation.
中文翻译:

钯催化内部炔烃的分子间反选择碳官能化为高度官能化的烯烃
本文已经建立了内部炔烃的钯/ DPEphos催化的分子间反选择性碳官能化。该方法通过正式的抗碳链缩合反应,形成反式链烯基钯物质,被芳基硼酸捕获,以32-92%的产率提供全碳四取代烯烃。内部炔烃的反式芳基甲硅烷基化/远程CH甲硅烷基化和加氢芳基化/远程CH甲硼化也分别使用六甲基乙硅烷和双(频哪醇)二硼作为捕获剂实现。该反应具有良好的区域选择性和立体选择性以及高官能团耐受性。初步的机理研究和DFT计算表明,顺式为顺式异构化-烯基钯物质参与该转化。
更新日期:2020-09-20
中文翻译:

钯催化内部炔烃的分子间反选择碳官能化为高度官能化的烯烃
本文已经建立了内部炔烃的钯/ DPEphos催化的分子间反选择性碳官能化。该方法通过正式的抗碳链缩合反应,形成反式链烯基钯物质,被芳基硼酸捕获,以32-92%的产率提供全碳四取代烯烃。内部炔烃的反式芳基甲硅烷基化/远程CH甲硅烷基化和加氢芳基化/远程CH甲硼化也分别使用六甲基乙硅烷和双(频哪醇)二硼作为捕获剂实现。该反应具有良好的区域选择性和立体选择性以及高官能团耐受性。初步的机理研究和DFT计算表明,顺式为顺式异构化-烯基钯物质参与该转化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号