当前位置:
X-MOL 学术
›
J. Label. Comp. Radiopharm.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Carbon‐13 synthesis and NMR spectroscopic geometric isomer evaluation to support the filing of teriflunomide
Journal of Labelled Compounds and Radiopharmaceuticals ( IF 0.9 ) Pub Date : 2020-08-31 , DOI: 10.1002/jlcr.3876 Michael Kurz 1 , Dirk Gretzke 1 , Rolf Hörlein 2 , Sandrine Turpault 3 , Jens Atzrodt 4 , Volker Derdau 1
Journal of Labelled Compounds and Radiopharmaceuticals ( IF 0.9 ) Pub Date : 2020-08-31 , DOI: 10.1002/jlcr.3876 Michael Kurz 1 , Dirk Gretzke 1 , Rolf Hörlein 2 , Sandrine Turpault 3 , Jens Atzrodt 4 , Volker Derdau 1
Affiliation
|
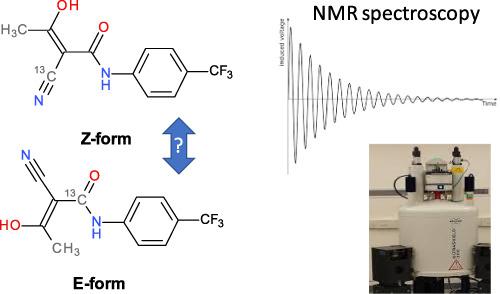
The two isotopomers of teriflunomide were synthesized starting from isotopically stable labelled stocks of [13 C]potassium cyanide and [1-13 C]ethyl bromoacetate. The two 13 C-labelled compounds 1a, b were applied in several NMR studies to study the E/Z ratio in different matrices. In solution, such as DMSO, a dynamic equilibrium between E/Z-isomers (ratio of 8:92) was determined by initial 13 C-carbon NMR experiments. To get insights into the E/Z ratio of teriflunomide under "in-vivo" conditions, advanced heteronuclear NMR (HOESY) in D2 O and mixtures of D2 O/plasma were performed. While NMR experiments in mixtures of water and plasma failed due to extreme line broadening, NMR spectra in water at pH 7.4, showed only the Z-isomer.
中文翻译:

碳 13 合成和核磁共振光谱几何异构体评估支持特立氟胺的备案
特立氟胺的两种同位素异构体是从[13 C]氰化钾和[1-13 C]溴乙酸乙酯的同位素稳定标记储备开始合成的。两种 13 C 标记的化合物 1a、b 被应用于多项 NMR 研究,以研究不同基质中的 E/Z 比。在溶液中,例如 DMSO,E/Z-异构体之间的动态平衡(比例为 8:92)由初始 13 C-碳 NMR 实验确定。为了深入了解“体内”条件下特立氟胺的 E/Z 比,在 D2O 和 D2O/血浆的混合物中进行了先进的异核 NMR (HOESY)。虽然水和等离子体混合物中的 NMR 实验由于谱线极端展宽而失败,但在 pH 7.4 的水中的 NMR 光谱仅显示 Z 异构体。
更新日期:2020-08-31
中文翻译:

碳 13 合成和核磁共振光谱几何异构体评估支持特立氟胺的备案
特立氟胺的两种同位素异构体是从[13 C]氰化钾和[1-13 C]溴乙酸乙酯的同位素稳定标记储备开始合成的。两种 13 C 标记的化合物 1a、b 被应用于多项 NMR 研究,以研究不同基质中的 E/Z 比。在溶液中,例如 DMSO,E/Z-异构体之间的动态平衡(比例为 8:92)由初始 13 C-碳 NMR 实验确定。为了深入了解“体内”条件下特立氟胺的 E/Z 比,在 D2O 和 D2O/血浆的混合物中进行了先进的异核 NMR (HOESY)。虽然水和等离子体混合物中的 NMR 实验由于谱线极端展宽而失败,但在 pH 7.4 的水中的 NMR 光谱仅显示 Z 异构体。











































 京公网安备 11010802027423号
京公网安备 11010802027423号