当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and in vitro study of new coumarin derivatives linked to nicotinonitrile moieties as potential acetylcholinesterase inhibitors
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2020-08-24 , DOI: 10.1002/jhet.4134 Ahmed E. M. Mekky 1 , Sherif M. H. Sanad 1
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2020-08-24 , DOI: 10.1002/jhet.4134 Ahmed E. M. Mekky 1 , Sherif M. H. Sanad 1
Affiliation
|
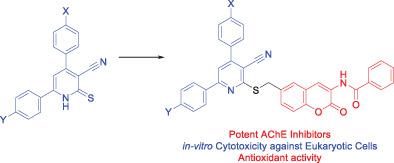
The appropriate pyridine‐2(1H)‐thiones were reacted with an equivalent amount of 5‐(chloromethyl)‐2‐hydroxybenzaldehyde in ethanol in the presence of potassium hydroxide to give the corresponding 2‐hydroxybenzaldehyde derivatives in excellent yields. The latter derivatives were taken as key synthons for the preparation of the target hybrids. Therefore, 2‐hydroxybenzaldehydes were reacted with benzoylglycine in acetic anhydride in the presence of fused sodium acetate at 100°C for 6 hours to afford a new series of nicotinonitrile‐coumarin hybrids. The in vitro acetylcholinesterase inhibitory activities were estimated for the new coumarins. The results were expressed as the inhibition percentage of the tested hybrids at concentration of 25 nM, compared to donepezil as a reference (inhibition percentage of 70.5). Coumarin hybrids linked to 6‐(4‐nitrophenyl) or 6‐(4‐chlorophenyl)‐4‐phenylnicotinonitrile exhibited more effective inhibitory activities than donepezil with inhibition percentages of 94.1 and 72.3, respectively. The new coumarins were tested for their free radical‐scavenging capabilities against DPPH. Furthermore, some new coumarins were tested for in vitro cytotoxic activity against each MCF‐10A, MCF‐7, Caco2, and HEPG2. The new hybrids showed cytotoxicity in micromolar range (IC50 of 3.5‐13.9 μM) against all tested cell lines. These results clearly demonstrated that the hybrids being tested are not cytotoxic at the concentration required to inhibit acetylcholinesterase effectively.
中文翻译:

与烟腈部分连接的香豆素衍生物作为潜在的乙酰胆碱酯酶抑制剂的合成及体外研究
合适的吡啶-2(1 H硫酮在氢氧化钾存在下与等量的5-(氯甲基)-2-羟基苯甲醛在乙醇中反应,以优异的收率得到相应的2-羟基苯甲醛衍生物。后一种衍生物被用作制备目标杂种的关键合成子。因此,将2-羟基苯甲醛与苯甲酰甘氨酸在乙酸酐中,在熔融乙酸钠存在下于100°C下反应6小时,从而得到一系列新的烟腈-香豆素杂化物。估计了新香豆素的体外乙酰胆碱酯酶抑制活性。结果表示为在25 nM浓度下测试的杂种的抑制百分比,与作为参考的多奈哌齐相比(抑制百分比为70.5)。与6-(4-硝基苯基)或6-(4-氯苯基)-4-苯基烟碱腈连接的香豆素杂种比多奈哌齐显示出更有效的抑制活性,抑制百分比分别为94.1和72.3。测试了新的香豆素对抗DPPH的自由基清除能力。此外,还测试了一些新的香豆素对每种MCF-10A,MCF-7,Caco2和HEPG2的体外细胞毒活性。新的杂种在微摩尔范围内显示出细胞毒性(IC3.5-13.9μM中的50))。这些结果清楚地表明,所测试的杂种在有效抑制乙酰胆碱酯酶所需的浓度下没有细胞毒性。
更新日期:2020-08-24
中文翻译:

与烟腈部分连接的香豆素衍生物作为潜在的乙酰胆碱酯酶抑制剂的合成及体外研究
合适的吡啶-2(1 H硫酮在氢氧化钾存在下与等量的5-(氯甲基)-2-羟基苯甲醛在乙醇中反应,以优异的收率得到相应的2-羟基苯甲醛衍生物。后一种衍生物被用作制备目标杂种的关键合成子。因此,将2-羟基苯甲醛与苯甲酰甘氨酸在乙酸酐中,在熔融乙酸钠存在下于100°C下反应6小时,从而得到一系列新的烟腈-香豆素杂化物。估计了新香豆素的体外乙酰胆碱酯酶抑制活性。结果表示为在25 nM浓度下测试的杂种的抑制百分比,与作为参考的多奈哌齐相比(抑制百分比为70.5)。与6-(4-硝基苯基)或6-(4-氯苯基)-4-苯基烟碱腈连接的香豆素杂种比多奈哌齐显示出更有效的抑制活性,抑制百分比分别为94.1和72.3。测试了新的香豆素对抗DPPH的自由基清除能力。此外,还测试了一些新的香豆素对每种MCF-10A,MCF-7,Caco2和HEPG2的体外细胞毒活性。新的杂种在微摩尔范围内显示出细胞毒性(IC3.5-13.9μM中的50))。这些结果清楚地表明,所测试的杂种在有效抑制乙酰胆碱酯酶所需的浓度下没有细胞毒性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号